Caught in the act: scientists discover how protein gives anticancer drugs the boot
March 4, 2014
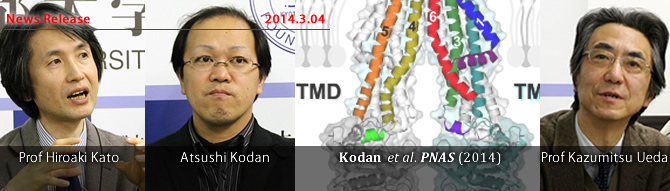
Kyoto, Japan -- Researchers in Japan have captured snapshots of how a protein responsible for chemotherapy resistance says no to drugs. Their findings, published in the Proceedings of the National Academy of Sciences, may one day help in the battle against cancer.The team of scientists, from Kyoto University's Institute for Integrated Cell Material Sciences (iCeMS) and Graduate School of Pharmaceutical Sciences, investigated the structure of a protein called CmABCB1, which is found in algae that live in highly acidic hot springs.
"CmABCB1 is located on the surface of cells and mainly operates by actively expelling a wide range of chemicals, thereby preventing an accumulation of toxic substances," said lead author Atsushi Kodan. "In human malignant tumors, a CmABCB1-like protein hABCA1, also called MDR1, is responsible for resistance against many structurally unrelated anticancer drugs."
After studying the protein's functional behavior, the researchers obtained protein crystals and succeeded in determining the 3D-structure of the protein at a high resolution. They discovered where drugs enter the protein's inner cavity and how they are exported out of cells.
"Importantly, we identified the drug entry and exit gates of CmABCB1 and how their opening and closing actions are powered by the protein to kick out drugs," said Kodan. "This is a unique mechanism."
"Ultimately, our findings may help to improve the design of drugs that will reach tumors at an effective concentration," said Kazumitsu Ueda, an iCeMS professor who was also involved in the study.
Publication Information
![]() Structural basis for gating mechanisms of a eukaryotic P-glycoprotein homolog
Structural basis for gating mechanisms of a eukaryotic P-glycoprotein homolog
Atsushi Kodana,1, Tomohiro Yamaguchib,1, Toru Nakatsub,c, Keita Sakiyamab, Christopher J. Hipolitod, Akane Fujiokab, Ryo Hirokaneb, Keiji Ikeguchie, Bunta Watanabee, Jun Hiratakee, Yasuhisa Kimuraf, Hiroaki Sugad, Kazumitsu Uedaa,f, and Hiroaki Katob,c,2
Proceedings of the National Academy of Sciences of the United States of America (PNAS) | Published Online 4 March 2014 | DOI: 10.1073/pnas.1321562111
- Institute for Integrated Cell-Material Sciences (WPI-iCeMS)
- Department of Structural Biology, Graduate School of Pharmaceutical Sciences, Kyoto University, Kyoto 606-8501, Japan;
- RIKEN Harima Institute at SPring-8, Hyogo 679-5148 Japan
- Department of Chemistry, Graduate School of Science, The University of Tokyo, Tokyo 113-0033, Japan
- Institute for Chemical Research, Kyoto University, Kyoto 611-0011, Japan
- Division of Applied Life Sciences, Graduate School of Agriculture, Kyoto University, Kyoto 606-8502, Japan
- A.K. and T.Y. contributed equally to this work.
- To whom correspondence should be addressed.
