Micropores are Here to Help! Welcome to the world of PCP/MOFs
The discovery of porous coordination polymers (PCP/MOFs) in 1997 by Susumu Kitagawa, now the director of iCeMS, was a breakthrough in materials science. With innumerable micropores arrayed in regular patterns, these materials have the potential to transform many aspects of our lives, ranging from environmental protection, energy, medicine, aerospace and manufacturing. In this article, two leading researchers will guide us into the World of PCP/MOFs.
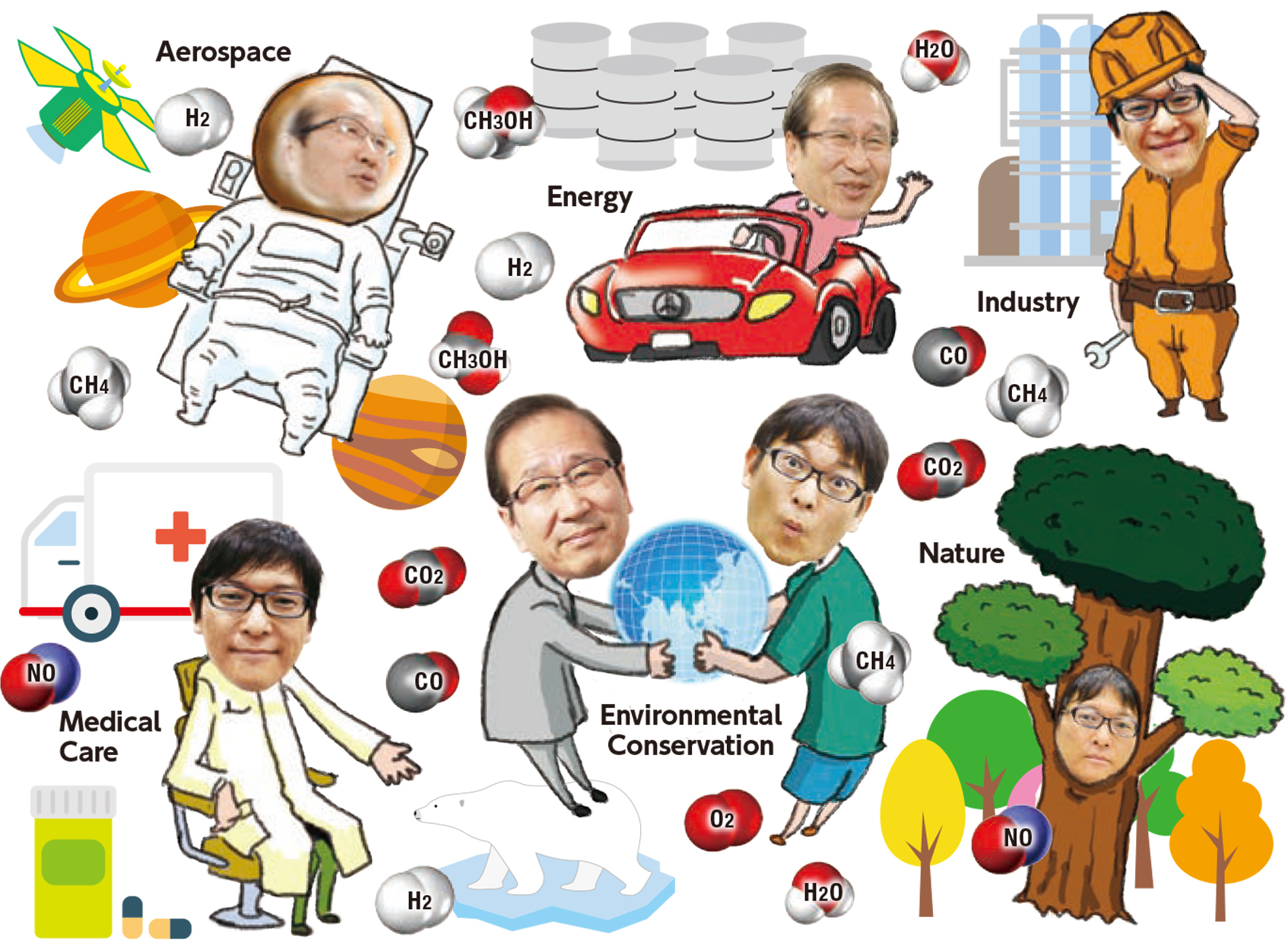
Realms Where PCP/MOFs Have Significant Roles
Susumu Kitagawa / Director
oremost PCP/MOF Researcher
Susumu Kitagawa
Born in 1951 in Kyoto. Received a PhD in Chemistry from the Graduate School of Engineering of Kyoto University. Held the positions of Associate Professor at the Faculty of Science and Engineering of Kindai University, Professor at the Department of Chemistry of Tokyo Metropolitan University, Professor at Graduate School of Engineering of Kyoto University, and Professor at iCeMS. Has been the Director of iCeMS since 2013. He was selected as a Thomson Reuters Citation Laureate in 2010, and received the Medal with Purple Ribbon in 2011.

Masakazu Higuchi / Program-Specific Assistant Professor
Aiming to create as-yet unknown value with PCP/MOFs
Masakazu Higuchi
Born in 1975 in Kochi, Japan. Completed his PhD in chemistry at the Graduate School of Engineering of Kyoto University in 2005. Held positions at RIKEN and the University of Tokyo, and has been at his present position since 2010. Started the venture company Atomis Inc. in 2015 aiming to accelerate the practical application of PCP/MOFs. Also enthusiastically engages in activities to communicate information about science-business related topics to the public and children.

The iCeMS Perspective on PCP/MOFs
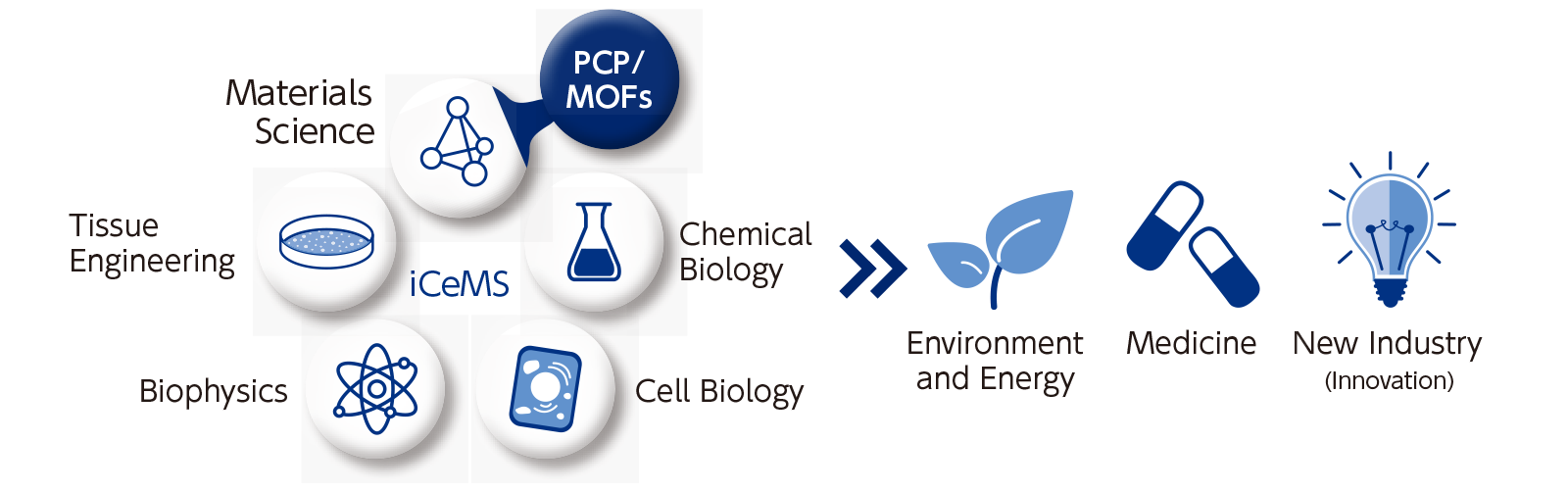
iCeMS is an interdisciplinary group of cell biologists, chemists and physicists, working from many viewpoints to address contemporary problems, such as global warming, pollution, disease and aging.
Currently within the field of materials science, PCP/MOFs have the potential to intersect with various fields in the future.
The History of Porous Materials
PCP/MOFs are a type of porous material. As the name suggests, porous materials contain empty spaces. Riddled with innumerable micropores, these materials may seem unserviceable at first glance, but, in fact, they have long been utilized in familiar ways. The following are some examples of porous materials, with a review of their history.
Approx 3500 years ago
1500BC
Activated Carbons
Today, activated carbons are used in many common products, including refrigerator and car deodorants and decolorization agents. They have also been connected with human life since ancient times. Records from ancient Egypt show activated carbons used for water purification and medical treatment. Activated carbons are made from vegetable materials such as coconuts. In these types of products, the pores are not uniformly arranged.


Approx 260 years ago
1756 Discovered in natural rock
1862 Composed artificially
Zeolite
Zeolites are a type of microporous mineral that exhibit roughly 220 different structural frameworks. Their uses include catalysis, ion exchange and gas purification, and they are especially important in petrochemical manufacturing. Zeolites have a very rigid structure made up mainly of silicon, aluminum and oxygen, with regularly arrayed micropores. Although the mineral framework can differentiate molecules by size, it cannot differentiate molecules close in size with similar properties. For this reason, it is difficult to use zeolite for precise adsorption and separation.


Although activated carbons and zeolites are indispensable for modern life, materials with even greater performance and energy efficiency are still needed.
Enormous amounts of energy are consumed in daily life, transport, manufacturing and other industries, and of this, 14% is spent on the process of separating chemical products. Development of porous materials may be the key to reducing the energy requirements for efficient storage, separation or conversion of target chemicals (gases and small molecules).
Meanwhile, an innovative material has appeared.
The name is…
20 years ago
1997
CP : Porous Coordination Polymer
also known as
MOF : Metal Organic Framework
Porous coordination polymers (PCP/MOFs) are materials discovered by Professor Susumu Kitagawa. Containing innumerable micropores of uniform size, they can take in many times more molecules than activated carbons and require little energy for efficient separation performance. In addition, they have 100 times more variations of pores than zeolite. Pore size and characteristics can be freely controlled by a combination of metal ions and organic ligands. Research on these materials began a mere 20 years ago, and further applications are expected expand into a wide range of fields.
Properties of PCP/MOFs
Porous coordination polymers are attractive materials that bring together all of the advantages of preexisting porous materials. Introduced here are three key properties that reveal the secrets of their performance.
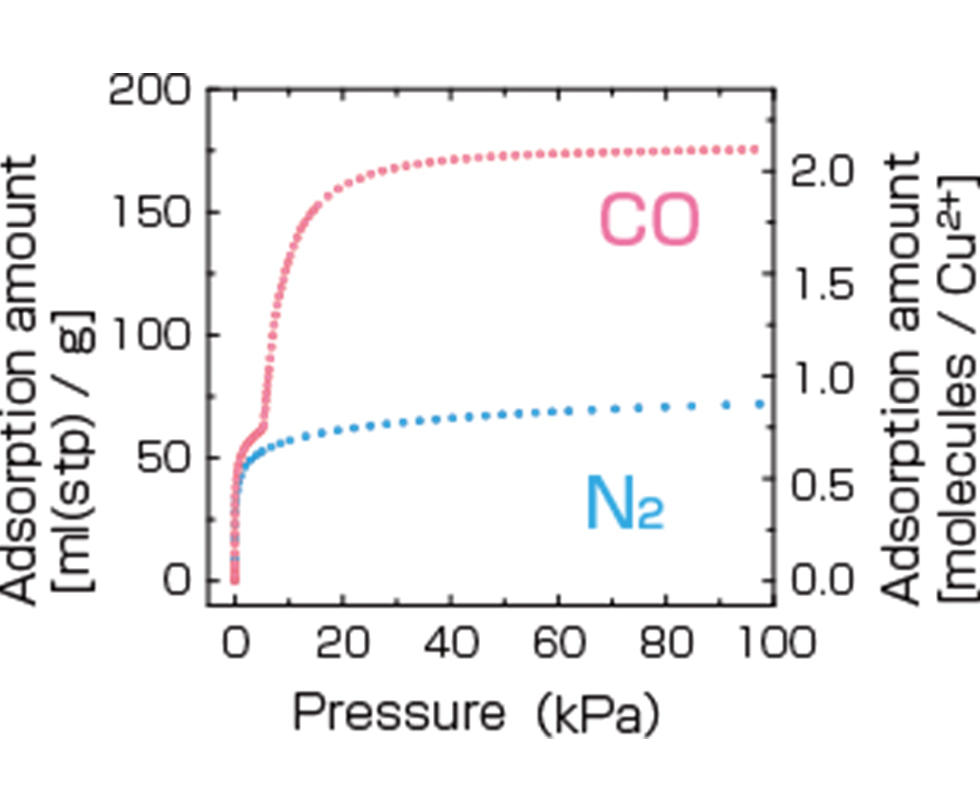
Large Surface Area
The larger the surface area, the more molecules adsorbed and the easier for reactions to occur. In other words, larger surface area means higher performance. Activated carbons also have large surface area, but they can’t match PCP/MOFs. A single gram of MOF material has a total surface area comparable to a soccer field!
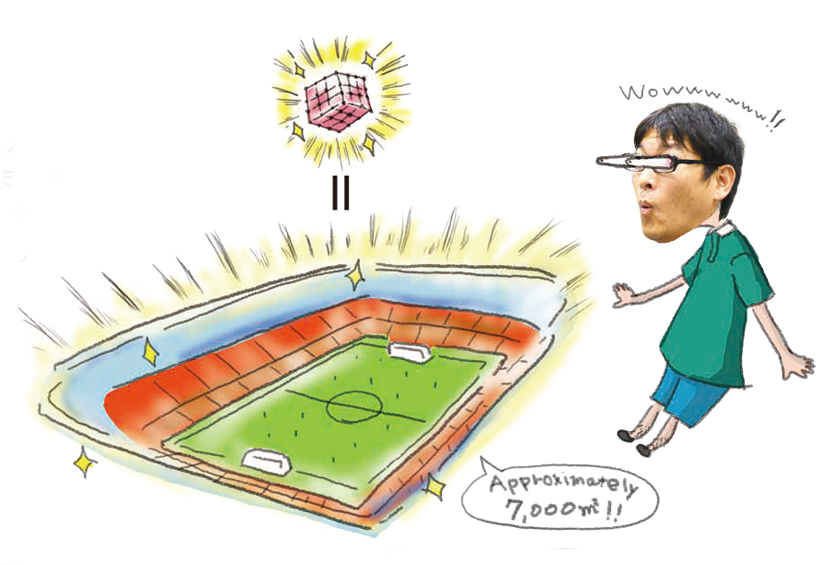
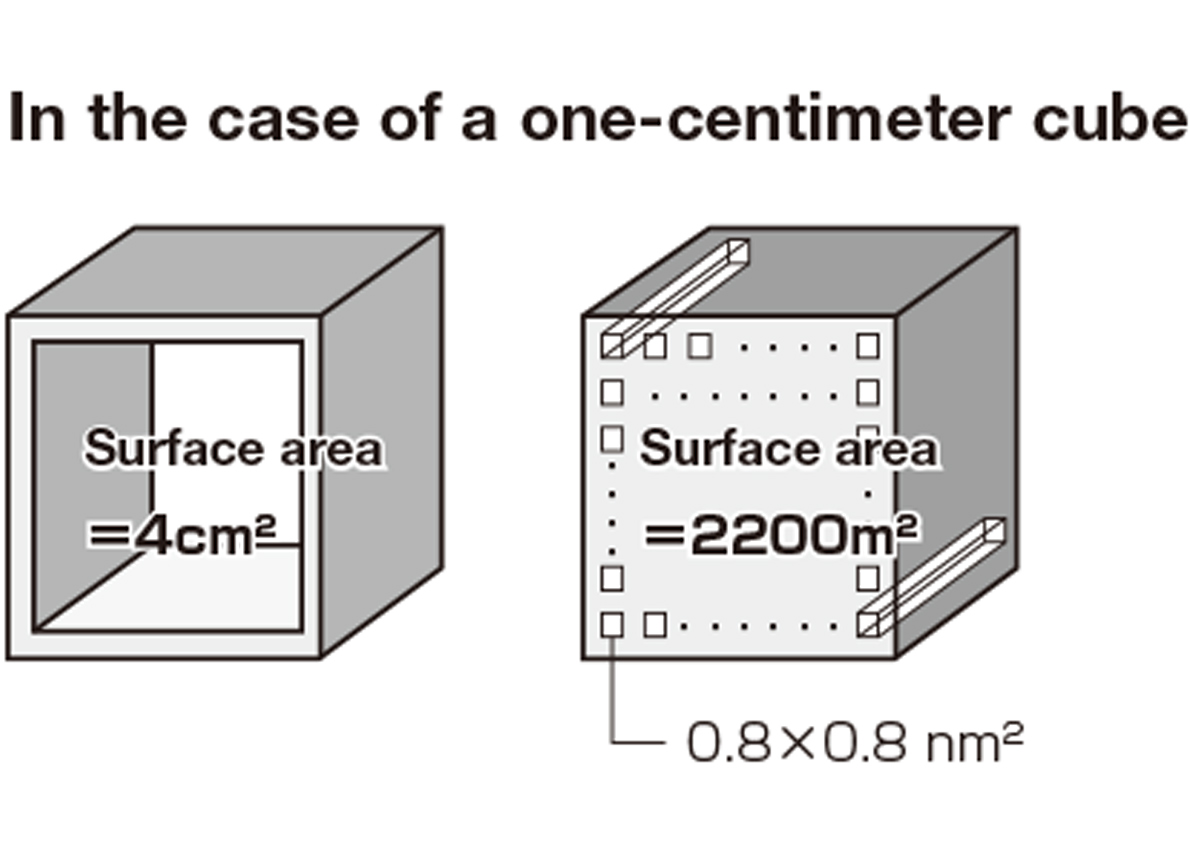
Imagine a cube that is one centimeter on each side. If a hole of 1 cm2 is bored through it, four internal surfaces will form. In this case, the surface area would be 4 cm2. Now imagine that a series of tiny holes, measuring one nanometer on each side, are drilled through that same one-centimeter cube. The interior surface area would be tremendously increased. Each pore hole is very small, but PCP/MOFs contain an enormous numbers of them.
Designability
PCP/MOFs are a continuous series of “metal complexes”, in which metal ions and organic ligands are connected by coordinate bonds. There are about 30 kinds of metal ions, and innumerable kinds of organic ligands, presenting limitless possible combinations. To date, 23,000 variations on the basic framework have been created, which represents a mere 20 years of research. As you read this, new PCP/MOFs may be newly synthesized in some laboratories.

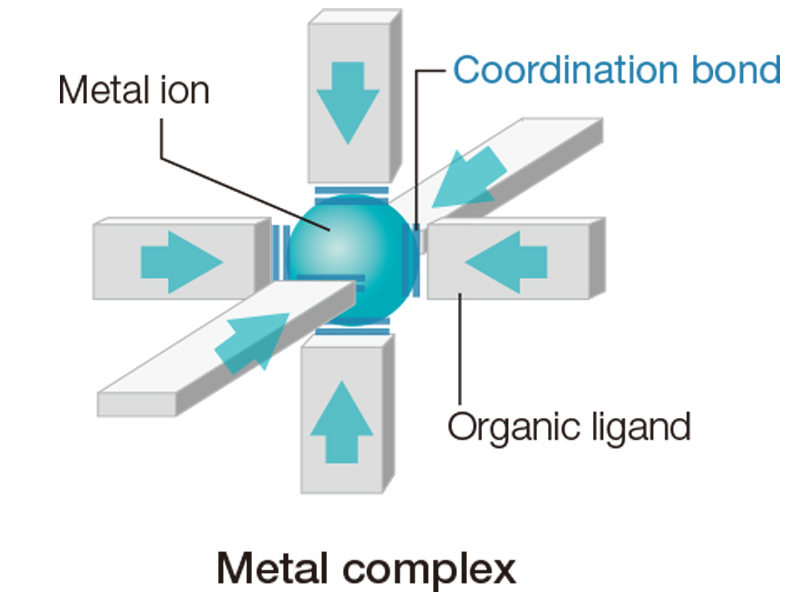
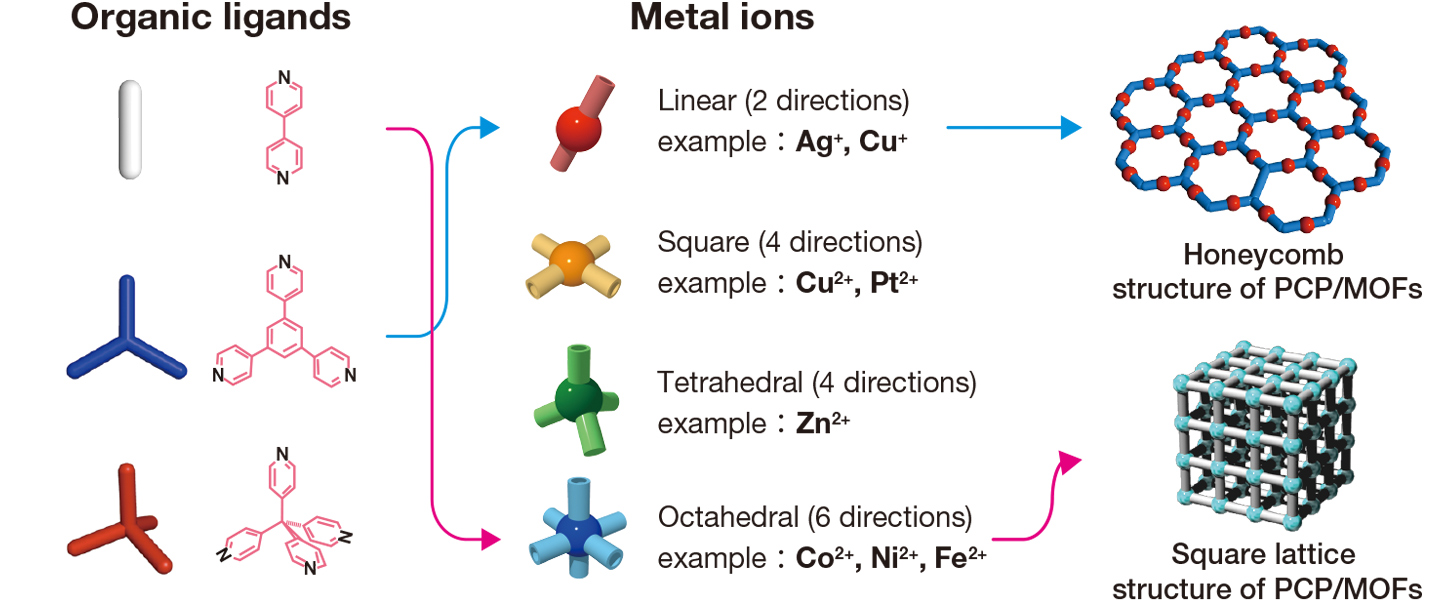
Metal ions are connectable in different directions, depending on their type. Some can connect in two directions, others in four directions. As the metal ions connect to both ends of organic ligands, a huge variety of structures is possible.
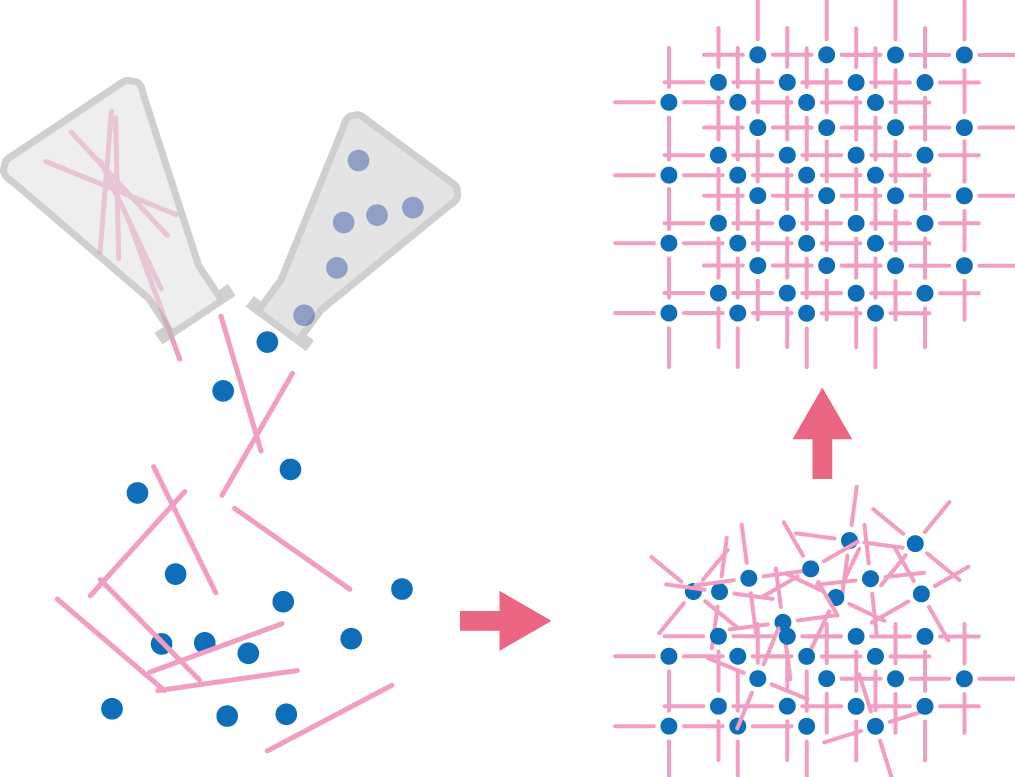
Ease of Fabrication
How do we actually make PCP/MOFs with metal complexes in endless series? The answer is simple: Mix an organic ligand solution with a metal ion solution. That’s all there is to it. Information is added, based on a design prepared in advance to instruct the ions to connect with both ends of the ligands. From this, the material is automatically generated.

What Can PCP/MOFs do? —Research Trends
PCP/MOF material development could not be described without mentioning three key functions: (1) Storage, (2) Separation, (3) Conversion. The main research development has focused on these three functions since work started in 1997. Tracing its history, the field has shifted over time from simple to complex research, as scientists took on ever greater challenges.
Storage
Pores in PCP/MOFs will adsorb and hold large quantities of molecules. A great deal of research has looked at the adsorption of hydrogen, methane, carbon dioxide and other gases, all for the ultimate purpose of storage. Early PCP/MOF research focused on storage considerations, such as how to maximize the number of molecules adsorbed and how to minimize the space needed to hold them.
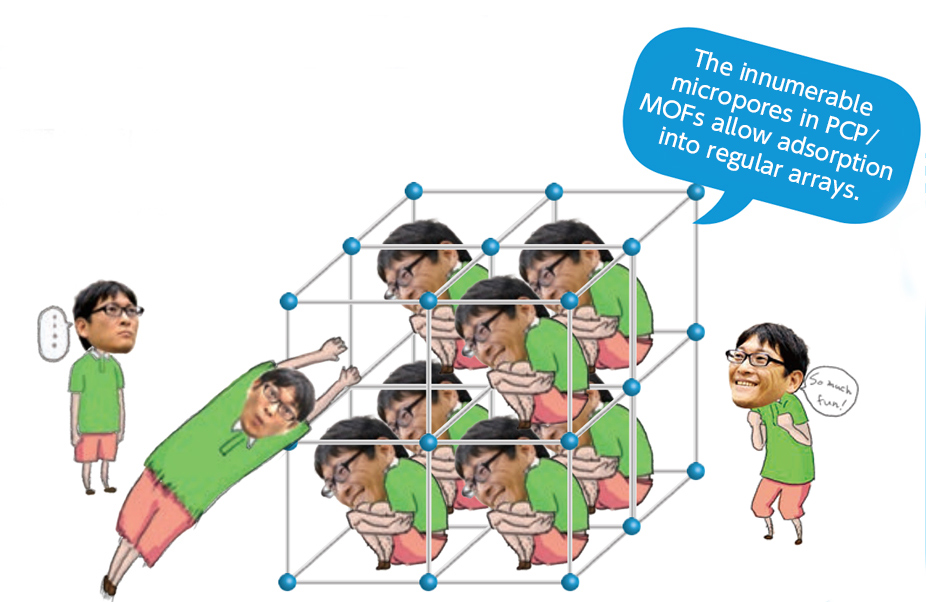
Separation
In substances containing several kinds of molecules, one molecule can be selected for adsorption into PCP/MOFs.
In activated carbon, the pores are not of uniform size, so various different molecules will be adsorbed at the same time. In PCP/MOF material, where the holes are all the same size, only a single target substance is adsorbed, producing outstanding separation performance.
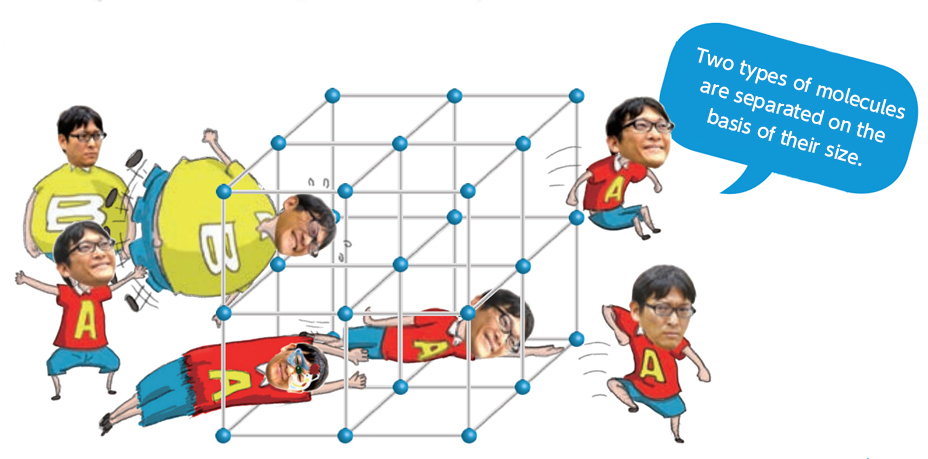
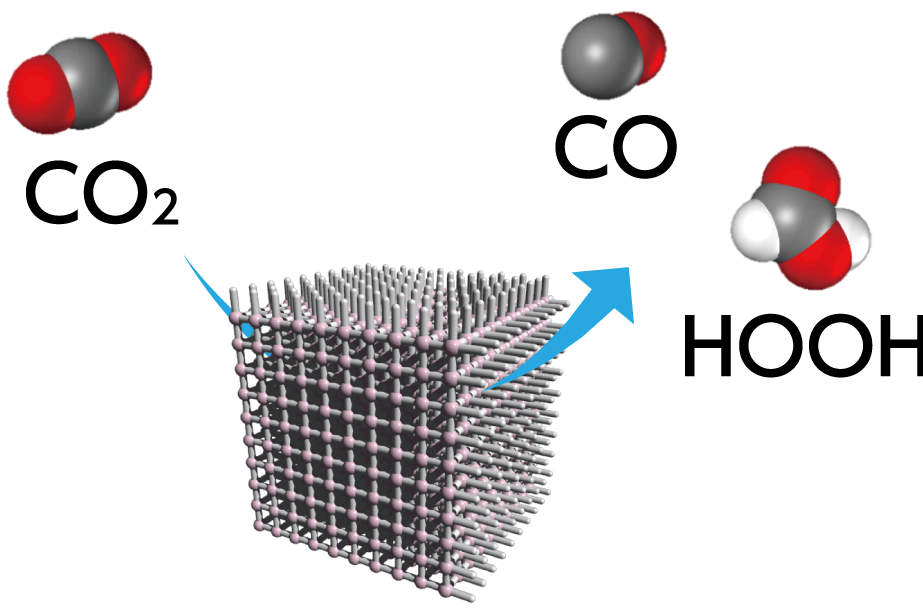
Conversion
One kind of molecule adsorbed into PCP/MOFs can be converted into a different type of molecule. When a highly reactive metal ion is incorporated in the framework of the material, it can work as a catalyst, stimulating a chemical reaction that converts the adsorbed molecules into another kind of molecule. An as-yet unobtained goal using this method is the conversion of environmentally harmful substances into useful substances. One such nuisance molecule is carbon dioxide, which causes global warming and abnormal weather patterns. PCP/MOF materials present a hope for some sort of alchemical miracle, such as converting carbon dioxide to methane.
Real-Life Applications of PCP/MOFs
Practical utilization of PCP/MOFs has so far been limited to only a few fields, but an enormous potential for crossover into other fields exists.
Two Commercial Examples
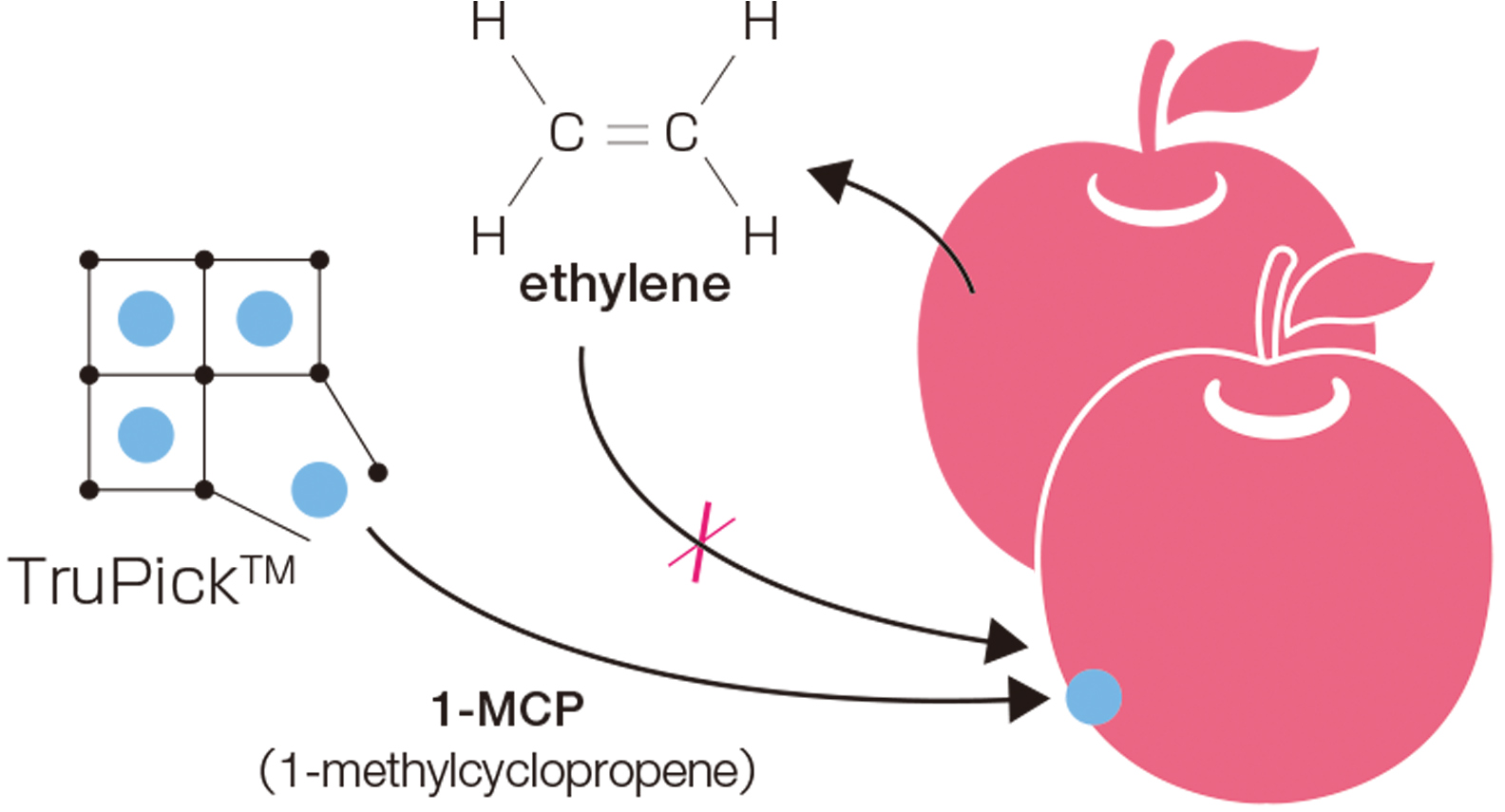
TruPick™
[ MOF Technologies, United Kingdom ]
September 2016 The world’s first commercial PCP/MOF product
Currently, approximately 40% of fruit spoils between the harvest and the consumer. This is because fruit releases ethylene, which adheres to the fruit surface and accelerates ripening.
TruPickTM is a packaging product that releases 1-methylcyclopropene (1-MCP), a chemical that inhibits the production of ethylene. It utilizes water-sensitive PCP/MOFs, breaking down when contacting moisture in the fruit. This causes 1-MCP to adhere to the packaged fruit, slowing ripening and decay and preserving freshness during transport and storage.
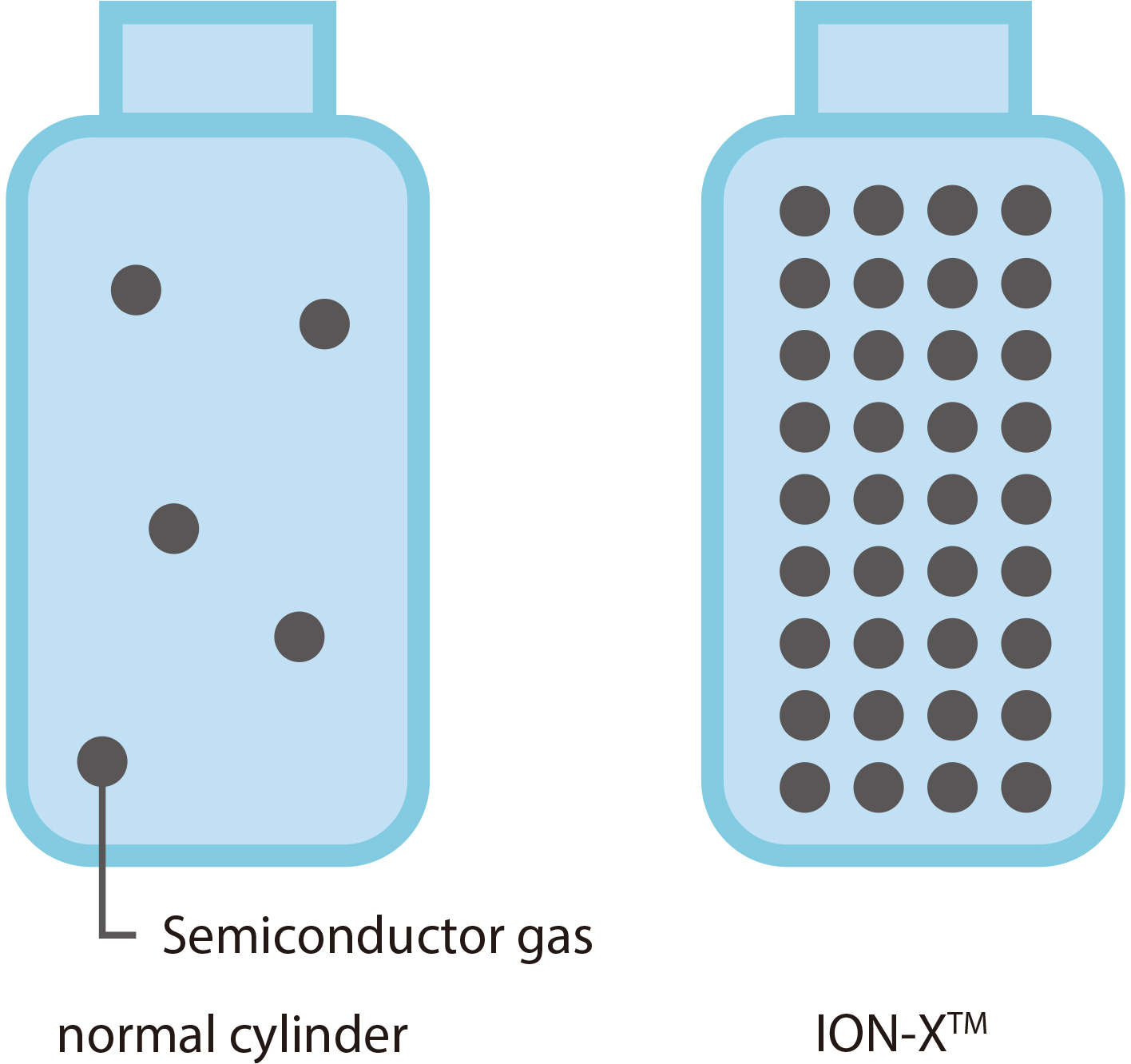
ION-X™
[ NuMat Technologies, USA ]
October 2016 Safe transport of hazardous gases
ION-XTM is a compressed gas cylinder that utilizes PCP/MOF storage functionality. A special kind of PCP/MOFs placed inside the cylinder allows gas storage at pressures several times lower than normal atmospheric pressure. If hazardous gases are stored at high pressure, they may leak out of the cylinder, an extremely dangerous event. By reducing pressure, ION-XTM enables safe transportation. NuMat Technologies is developing a gas delivery business serving semiconductor factories in South Korea.
Further promise...
R&D for Innovative Filters
March 2018 Joint R&D by Dr. Higuchi and a company in Kyoto
Various deodorant products have long helped eliminate the unpleasant odors of toilets, tobacco smoke, etc. However, existing products do not remove all the odor molecule, leaving a portion of the unwanted smell. Dr. Higuchi from iCeMS, working together with Ohara Paragium Chemical Co., Ltd. is developing PCP/MOFs capable of instantly and completely eliminating odors. Still in the development stage, this work could lead to more comfortable living and work space in the near future.

Venture companies connecting universities and large companies
Venture companies play two roles in developing new materials: creating materials by applying basic university research and providing materials to large companies. To date, many venture companies have put new materials into practical use. Unfortunately, in Japan, there is not much of a mechanism for relating basic research to society. In 2015, Dr. Higuchi founded the venture company, “Atomis Inc.”, which specializes in mass production of PCP/MOFs and the creation of new materials. The company has tried to be a bridge between universities and large companies.
Universities are responsible for fundamental research; venture companies are responsible for the “bridge”; and large companies are responsible for wide distribution. To develop new materials, these three roles are indispensable. In developing new materials, it is essential that people with diverse backgrounds approach the same goal by using their diverse strengths.
Published in November 2019 Reprinted from iCeMS Our World, Your Future vol.7
Production cooperation: Kyoto Tsushinsha