Two-organ chip to answer fatty liver questions
A tiny platform empowers scientists to examine how gut and liver cells interact in health and disease.
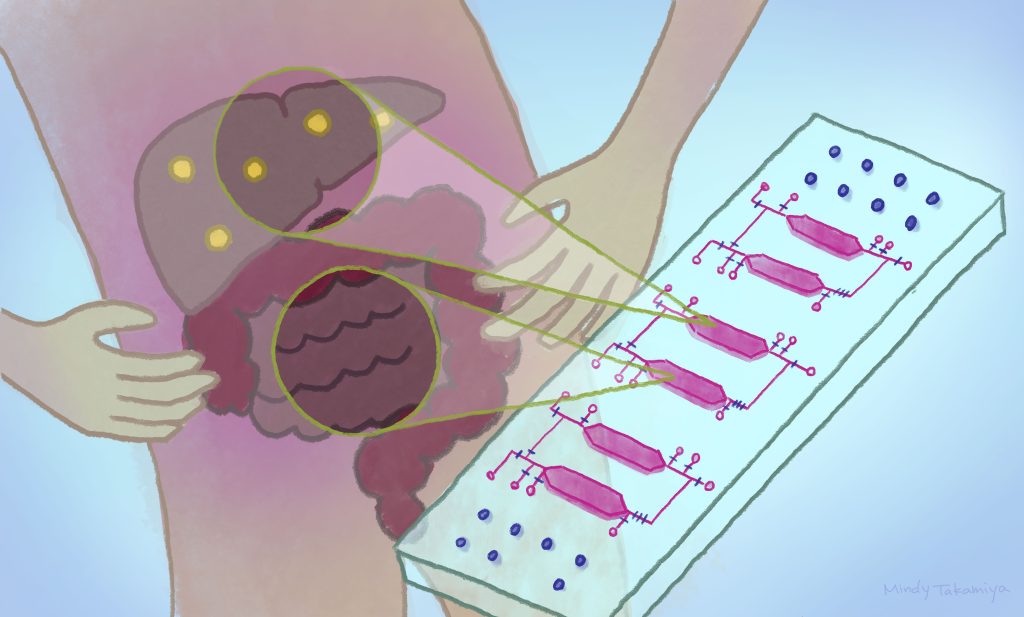
A new chip that holds different cell types in tiny, interconnected chambers could allow scientists to better understand the physiological and disease interactions between organs. The integrated-gut-liver-on-a-chip (iGLC) platform was designed by scientists at Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS), to improve understanding of non-alcoholic fatty liver disease (NAFLD). The researchers, together with colleagues in Japan, published their findings in the journal Communications Biology.
“NAFLD affects a significant percentage of the population, but no effective treatments have been established,” explains iCeMS bioengineer Ken-ichiro Kamei, who led the study. This is because NAFLD is a complex condition, involving a wide range of interactions inside and between the gut and the liver, known as the gut-liver-axis. It is very difficult to model these interactions using animals, such as mice, due to the many differences between species.
NAFLD involves the build-up of fat inside the liver, which can become severe. Currently, the only way to treat severe cases is with liver transplantation. Scientists need better approaches to study the condition to be able to discover improved options for treatment.
This isn’t the first time scientists have developed organ-on-a-chip platforms, nor is it the first gut-liver platform, but previous devices have been imperfect. The platform developed by Kamei and his colleagues overcomes some but not all the issues with previous attempts.
The scientists tested their iGLC platform by placing cells from a liver cancer cell line and from a gut cancer cell line into separate chambers. The chambers are connected by tiny fluidic channels with strategically positioned valves that can be opened and closed. The platform also includes a pump for pushing fluid between the chambers. They allow a fluid medium to pass through both chambers while keeping the cells separate, mimicking the circulation moving between the gut and liver in the human body. It also allows the scientists to introduce new substances into the platform for example free fatty acids to test their impacts on the two interacting “organs”.
Importantly, the platform is made of a silicone material, called polydimethylsiloxane (PDMS), that is coated with two other substances: one that prevents the chip from absorbing fatty molecules that could affect experiments, and another that increases cell growth.
Significant changes in gene expression were seen in gut and liver cells cultured in the iGLC platform when compared to the same cells cultured on their own. The scientists also documented the specific changes that happened in the cells when free fatty acids were introduced for one or seven days. One day of free fatty acids led to the initiation of DNA damage inside the cells. Seven days of circulating free fatty acids led to their accumulation in the cells to the point that the DNA damage led to cell death, similar to severe cases of NAFLD.
The platform does not take into account the impacts of gut microbes or other factors on the gut-liver-axis. The experiments also used cancer cell lines, which don’t represent the full diversity or functionality of cells in living human tissue, but the platform is a significant step forward.
“We next plan to use liver and gut organoids derived from human stem cells so we can investigate NAFLD under precisely controlled conditions that more closely mimic patients’ physiological contexts,” says iCeMS mechanical engineering researcher, Yoshikazu Hirai, the study’s corresponding author.
Paper Information
“Integrated gut–liver-on-a-chip platform as an in vitro human model of non-alcoholic fatty liver disease”
Authors:Jiandong Yang, Yoshikazu Hirai, Kei Iida, Shinji Ito, Marika Trumm, Shiho Terada, Risako Sakai, Toshiyuki Tsuchiya, Osamu Tabata, and Ken-ichiro Kamei