Once Thought Rigid: Unveiling the Unexpected Softness of the First PCP
Program-Specific Lecturer (Kitagawa Lab)
Hirotoshi Sakamoto
Hirotoshi Sakamoto
Porous Coordination Polymers (PCPs) are a new type of porous material that is currently attracting attention around the world. The first PCP was reported in 1997, demonstrating that it adsorbs significant amounts of gases. Dr Hirotoshi Sakamoto and his colleagues clarified that the gas adsorption of the iconic PCP in 1997 was progressing through a mechanism based on the “softness” of the local structure, which may overturn the conventional view of the history of PCP research.

Please share with us the significance of your manuscript in terms of its research achievements, impact, or uniqueness.
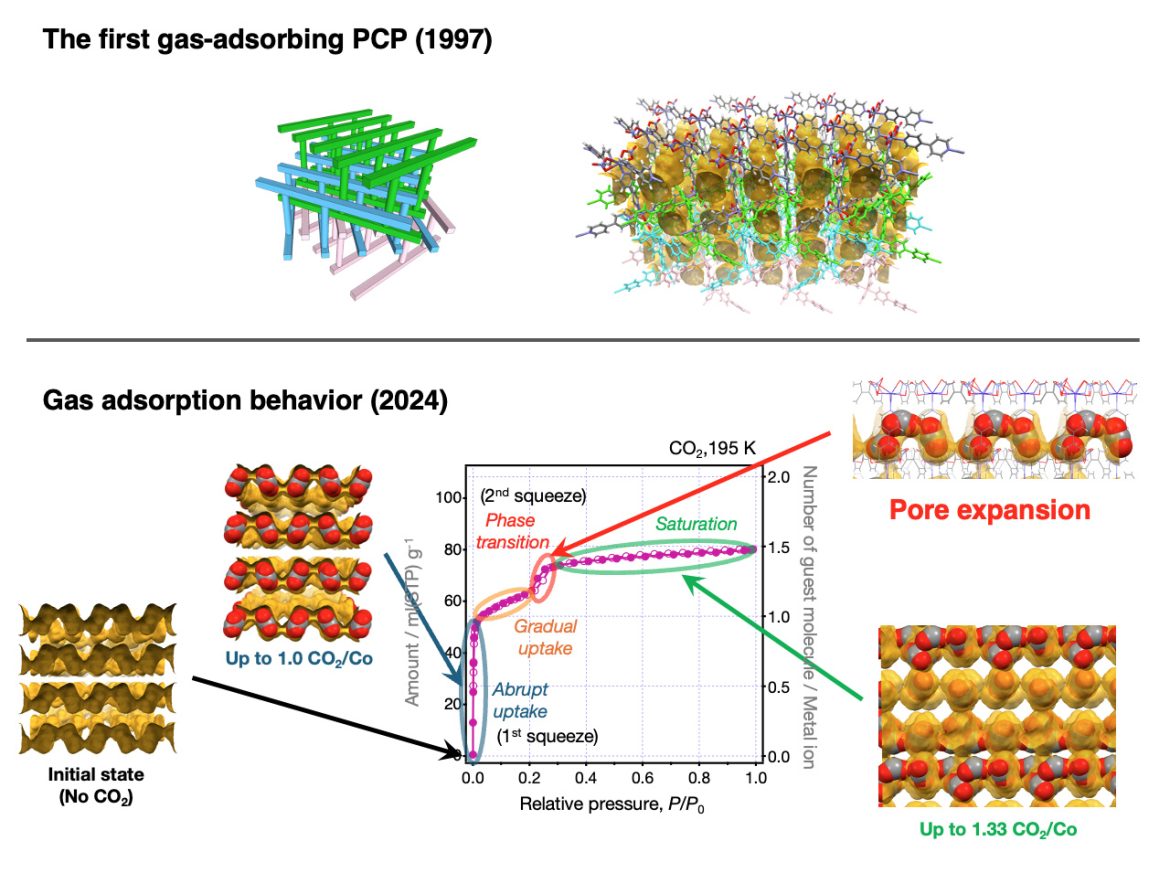
The “first gas-adsorbing PCP” was also the “first soft PCP.” The “first gas-adsorbing PCP” discussed in this paper was originally published in 1997 by Professor Susumu Kitagawa, co-author of this article. At that time, the prevailing belief was that porous materials capable of gas adsorption must have rigid frameworks. As a result, we too assumed that the framework of this PCP must be rigid. However, a lingering mystery remained: if the framework was indeed rigid, how could gas molecules pass through channels narrower than themselves? Thus, we began this study with the simple idea of using the latest technology to verify gas adsorption on this supposedly rigid framework. However, as our research progressed, we continuously obtained data that could not be explained by a rigid structure. This led us to conclude that the PCP must, in fact, be “soft.”
What is particularly interesting is that the concept of a “soft PCP” did not exist in 1997. This concept was proposed by Professor Susumu Kitagawa in 1998 for the first time, who also predicted its emergence. Considering how deeply rooted the belief in “gas adsorption = rigid framework” was at the time, it becomes clear how unconventional—and ultimately groundbreaking—this idea was. Just a few years later, flexible PCPs were successfully synthesized, and their remarkable properties became evident.
Returning to our current research, it turns out that, back in 1997, even before the concept was proposed or predicted, we had already encountered a “soft PCP.” While the history of soft PCPs began with our group, we now find ourselves rewriting the first page of that history with our own hands.
Please tell us what was the most gratifying or inspiring moment for you during this research project.
A key moment was when we were able to confirm the crystal structure of PCP with its pores expanded, by conducting X-ray diffraction measurements and structural analysis under high-pressure carbon dioxide.
As we continued our research, we encountered an issue that the amount of CO2 adsorbed on the isotherm did not match the amount expected from the “rigid framework structure”. To address this discrepancy, we performed crystal structure analysis under conditions where CO2 was forced into the PCP. This analysis revealed that the internal space of the PCP had expanded, allowing it to absorb more CO2 molecules than expected. When the behavior of the adsorption isotherm precisely matched the CO2 adsorption calculated from this new crystal structure, I was convinced that the long-standing mystery since the birth of PCP had finally been solved.
This unexpected findings provided decisive evidencethatPCP in this study was indeed a soft material.
Please tell us about the biggest challenge or problem you experienced while conducting your research. How did you overcome it?
When the PCP comes into contact with moisture in the air, it immediately transforms into a different crystalline structure that does not adsorb gas. This posed a significant challenge for us. Initially, we were unaware that this phenomenon was occurring, leading to variations in the gas adsorption amount for each sample, making it difficult to obtain reliable data. In fact, during my time as a master’s student, I also conducted research using this PCP but encountered this very problem, leaving me frustrated and unable to make progress. This time, however, we were able to identify the crystalline structure after the transformation and determine its cause. Additionally, we discovered a method to regenerate the original PCP. By carefully controlling each step, including washing, drying, and adsorption measurements, without exposing the synthesized or regenerated PCP to ambient air, we were finally able to obtain stable and reproducible data. This breakthrough brought us a great sense of relief.
Would you consider this work to be a turning point in your overall research direction? If so, how has your research direction changed as a result of this work?
By tackling unresolved issues from the past with the latest knowledge and technology, I realized that not only can we solve these problems, but we may also uncover even more remarkable new discoveries. The 1997 paper was a turning point that laid the foundation for the current PCP/MOF field, and this new paper serves as an update to that pioneering work, 27 years later. Our group originally pioneered the history of gas-adsorbing PCPs and soft PCPs, and having the opportunity to rewrite the first chapter of that history with our own hands once again carries great significance for us. While I cannot yet predict the impact this will have on my research career, I am certain that it will have a lasting impact on the PCP/MOF research field.
Please describe the current situation of your career. What is your current position? How has the knowledge and experience gained at iCeMS influencing your career?
I am currently a junior associate professor at iCeMS, where I am involved in multiple research projects simultaneously. My main focus is a project under NEDO’s (New Energy and Industrial Technology Development Organization) Green Innovation Fund, which aims to develop PCPs for the efficient capture of carbon dioxide from exhaust gases. In addition, I am pursuing personal research aimed at elucidating the adsorption behavior of single crystalline particles of MOF/PCP using X-ray absorption imaging. Recently, I have also taken on the role of supporting users of cutting-edge analytical instruments at the analysis center.
When iCeMS was established in 2007, I was a graduate student in Professor Kitagawa’s lab. I observed iCeMS from a slight distance and was profoundly influenced by its research style, which seeks originality through the fusion of different fields. After obtaining my degree, I worked at various research institutions, engaging in research across different fields, and this interdisciplinary research approach became a guiding principle for my work. Later, in 2022, I returned to Kyoto University and resumed my research at iCeMS. This gave me the opportunity to use the latest knowledge and technology to bring closure to the research which had not been accomplished in the past. Moving forward, I aim to maintain a multidisciplinary and integrative approach,pursuing research that, while not widely recognized, holds fundamental importance.
Paper information
“Progressive gas adsorption squeezing through the narrow channel of a soft porous crystal of [Co2(4,4’-bipyridine)3(NO3)4]”
Authors: Hirotoshi Sakamoto, Ken-ichi Otake, and Susumu Kitagawa
Communications Materials (Springer Nature) | DOI: 10.1038/s43246-024-00609-x
Published: September 2024