Invigorating Immunotherapy with Epigenetic Modulation
Postdoctoral Research Associate at University of Pittsburgh
PhD student (Sugiyama Lab and Namasivayam Lab), at the time working on this research
Madhu Malinee
Madhu Malinee
Dr Madhu Malinee was a PhD student at the Graduate School of Medicine of Kyoto University. She worked on her research with iCeMS Namasivayam and Sugiyama Labs, and left for her new position at the University of Pittsburgh this fall. During her PhD, she worked on improving the efficacy of immune checkpoint (PD-1) blockade-based immunotherapy to treat cancer. She designed an epigenetic modulator, EnPGC-1, that enhances mitochondrial functions of immune CD8+ T cells by activating PGC-1α/β coactivator. Furthermore, she found that in vivo EnPGC-1 synergizes with PD-1 blockade therapy and enhances tumor regression and survival of tumor bearing host.

Please share with us the significance of your manuscript in terms of its research achievements, impact, or uniqueness.
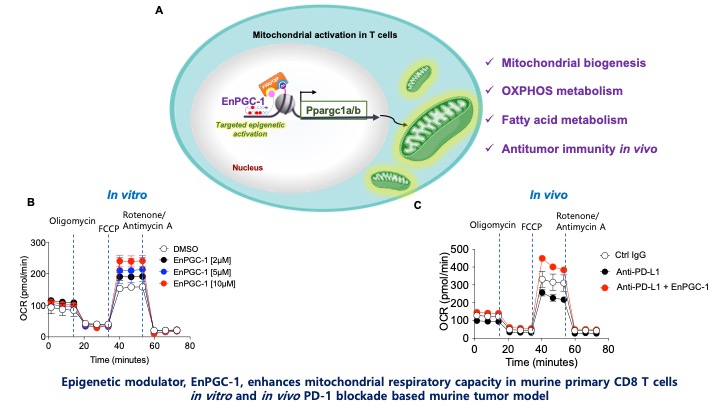
Cancer immunotherapy based on immune checkpoint blockade (especially PD-1 based) has shown a dramatic and beneficial effect for cancer treatment compared with other conventional therapies. However, a substantial fraction of patients do not respond to this therapy. I designed a programmable epigenetic modulator, EnPGC-1, that activates a coactivator, PGC-1, which enhances mitochondrial function and biogenesis of cancer-fighting immune cells. The immune cells boosted by EnPGC-1 live longer and kill the cancer cells more effectively. We showed the combination of designer molecule EnPGC-1 with PD-1 blockade improves the tumor regression and survival of the host compared to PD-1 blockade alone. Our programmable DNA-based epigenetic activator used in combination with PD-1 blockade-based cancer immunotherapy could open a new avenue to treat less-responsive cancer patients. EnPGC-1 is the first sequence-specific epigenetic ligand designed to enhance the mitochondrial functions of immune cells. Since EnPGC-1 improves mitochondrial function and mitochondrial respiration capacity, it may be fine-tuned for treating hyperlipidemia, type 2 diabetes, and other mitochondrial dysfunction-associated diseases.
Please tell us what was the most gratifying or inspiring moment for you during this research project.
I remember the most fascinating moment was when our compound worked successfully in the in vivo mouse tumor model. We successfully validated different aspects of EnPGC-1 functioning on primary murine immune CD8 T cells in various in vitro systems, however, we were not sure whether it would work in the in vivo system since many factors affect in vivo functions such as target specificity, retention time, and cross-reaction, etc. When we treated colon carcinoma-bearing mice with PD-1 blockade in combination with EnPGC-1, to our surprise, we found the combination therapy had significantly boosted the immune response and caused more tumor regression compared to PD-1 blockade alone. EnPGC-1 in combination with PD-1 blockade also improved the survival of the host. It was the most gratifying moment for me to know that my compound worked successfully in vivo. Showing that this designer molecule could have clinical benefits and the potential to be used to treat cancer patients was a major achievement for us. I can say that this moment deserves to be proclaimed as the “I made it” moment.
Please tell us about the biggest challenge or problem you experienced while conducting your research. How did you overcome it?
During this project, we wanted to have concrete evidence of the biological feasibility and effectiveness of EnPGC-1 functioning. It was difficult to decide which assay would show a direct correlation of measurable biological phenomenon to EnPGC-1 mediated PGC-1α/β upregulation. Because PGC-1 upregulation has a positive impact on mitochondrial biogenesis and respiration, we decided measuring mitochondrial respiration could give us the best observation of PGC-1 enhancement in immune cells. Since oxygen is the terminal electron acceptor in the electron transport system, the consumption of oxygen in the cells from the media could give a direct correlation. We measured the mitochondrial respiration by assessing the oxygen consumption using a Seahorse Analyzer, an instrument which measures the consumption of oxygen and the pH change in the culture plate. The measurement of mitochondrial respiration directly supported our hypothesis that EnPGC-1 enhances mitochondrial respiration by increasing PGC-1α/β.
Would you consider this work to be a turning point in your overall research direction? If so, how has your research direction changed as a result of this work?
In this research, we have shown for the first time the use of an epigenetic modulator to enhance cancer immunotherapy efficacy. Yes, I think this work is a turning point in my overall research direction. I believe this work is opening a new avenue for the development of an artificial biomimetic epigenetic modulator to effectively control the various immune response-associated genes to improve cancer immunotherapeutic efficacy. The knowledge gained during this research will be helpful in future research and this platform can be useful in designing epigenetic regulators for any gene that curbs disease, for example, treating type 2 diabetes, enhancing fatty acid oxidation associated key genes to treat hyperlipidemia, and enhancing TNF-α in killer cells to control cancer cells.
Please describe the current situation of your career. What is your current position? How has the knowledge and experience gained at iCeMS influencing your career?
iCeMS is one of the world-renowned research institutes. The high standard of research by scientists from various disciplines at iCeMS has set an example for the world. The novel high quality research there always inspired me and boosted my passion for conducting better scientific research. Several scientific meetings and interactive seminars hosted by iCeMS motivated and encouraged me to acquire the latest ideas for research. Technically advanced instruments at iCeMS helped me to design and perform my experiments in a feasible and easy way. The collaborative work environment involving various disciplines motivates and provides a common platform for students to learn from each other. I gained a lot of knowledge about immunotherapy approaches during my research. From this winter, I have my research as a postdoc at the University of Pittsburgh, Pittsburgh, USA.
*All the information on this page, including the researcher’s affiliation, is current at the time of the interview.
Paper information
Targeted Epigenetic Induction of Mitochondrial Biogenesis Enhances Antitumor Immunity in Mouse Model
Madhu Malinee, Ganesh Namasivayam Pandian and Hiroshi Sugiyama
Cell Chemical Biology
Published: September 2021
DOI: 10.1016/j.chembiol.2021.08.001