Synthesizing Porous Materials that can Absorb CO2 from CO2
Postdoctoral fellow at the University of Oregon studying under the JSPS Overseas Research Fellowship program
PhD student with the Horike Lab, at the time working on this research
Kentaro Kadota
Kentaro Kadota
While carbon dioxide is a cause of environmental problems, it is also a carbon resource that exists universally and abundantly on the earth. If we can convert carbon dioxide in the atmosphere into valuable chemicals and materials, it will lead to the realization of a sustainable society. Dr. Kadota and his research team have discovered a method to synthesize useful porous materials from carbon dioxide at ambient temperature and pressure.

Please share with us the significance of your manuscript in terms of its research achievements, impact, or uniqueness.
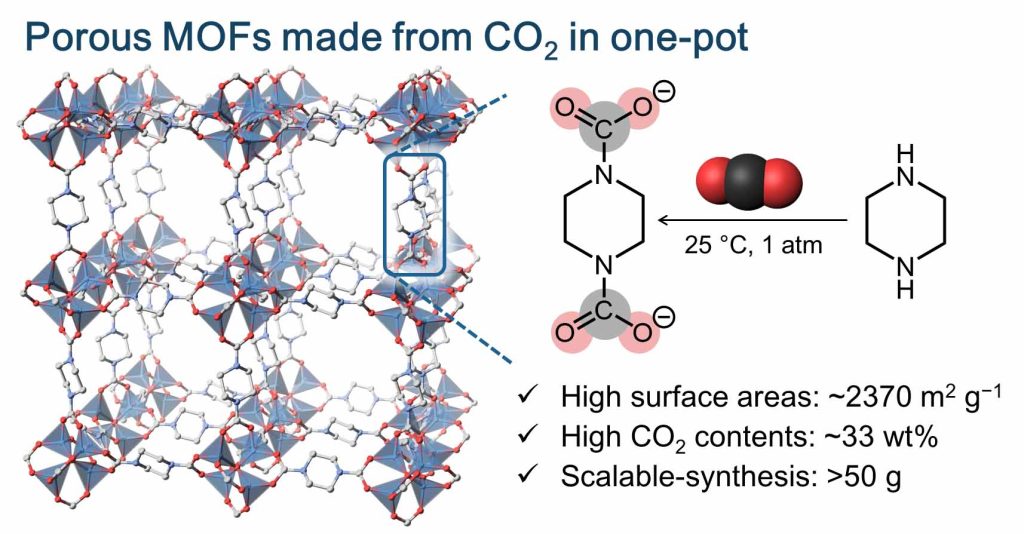
The key point of this research is that we were able to easily produce useful porous materials from carbon dioxide under mild conditions of ambient temperature and pressure.
While on one hand carbon dioxide (CO2) is a "nuisance" that is the cause of various environmental problems, it can also be seen as a "carbon resource" that exists in abundance everywhere on Earth. However, converting chemically inert CO2 into useful chemicals and materials has required precious metal catalysts, high temperature, and/or high pressure conditions.
Porous materials, such as the activated carbon and zeolite in water purifiers and air cleaners, are materials that have countless microscopic holes (pores) inside them. In recent years, research on porous materials has advanced, and they are being used in a wide range of fields from energy storage to gas separation.
In this study, for the first time we succeeded in making a porous metal-organic frameworks (MOF/PCP) from CO2 in a one-pot synthesis using amines under mild conditions of ambient temperature and pressure. The synthesized MOFs are more than 30% CO2 by weight and forms a stable pore structure which can store CO2 and H2. In addition, these MOFs can also be assembled directly from dry air by making use of the dilute CO2 found in the atmosphere (0.04%).
Please tell us what was the most gratifying or inspiring moment for you during this research project.
The most exciting moment for me was when I obtained the predicted powder X-ray diffraction (PXRD) pattern. PXRD is a technique that uses X-rays to examine the periodic structure of solids at the atomic and molecular level. When the structure is not neatly organized a pattern with almost no peaks is obtained (a disappointing result). For the first six months of my research, the patterns were almost completely lacking peaks and agreeability. Certainly, the happiest and most surprising moment of my research was when I obtained the very beautiful pattern I had expected when I hit upon the right reaction conditions.
Please tell us about the biggest challenge or problem you experienced while conducting your research. How did you overcome it?
We had the most difficulty finding the conditions for synthesizing a crystalline MOF from CO2. Until now the idea of making MOFs using CO2 was nonexistent, so there were no known synthesis or design guidelines that we could refer to. To solve this problem, we subdivided the process of making an MOF from CO2 into two steps: the reaction to convert CO2 into bridging ligands (the building blocks of MOFs), and the crystallization of the MOF structure. We then tried to optimize the conditions at each step. In the end, by reconciling the two steps, we were able to achieve a simple, one-pot synthesis of a MOFs from CO2 at ambient temperature and pressure.
Would you consider this work to be a turning point in your overall research direction? If so, how has your research direction changed as a result of this work?
This is related to my previous answer, but I learned that simplifying a seemingly complex problem into understandable parts can lead to a breakthrough. I believe that having had a taste of the joy of that comes from solving a real puzzle will be useful in my future research life. Also, after iCeMS made a press release of our research results, it was a very refreshing experience to receive various reactions and responses to from the media and public. As research tends to be confined to the laboratory, I think it was a valuable opportunity for us to recognize the importance of focusing on the return of research results to society.
Please describe the current situation of your career. What is your current position? How has the knowledge and experience gained at iCeMS influencing your career?
I am currently doing research at the Brozek Lab, University of Oregon, as a JSPS Overseas Research Fellow. One thing I learned at iCeMS is the importance of talking with people who have different perspectives and backgrounds. This is not just to get ideas you would never think of yourself. When you explain your research to someone with a different background, you must restructure the content which can make you see the subject and interest in a fresh light. I believe that this experience will be very useful as I begin work at a highly diverse laboratory in the US and in my future life as a researcher.
Finally, please give a message or advice to young iCeMS researchers.
At iCeMS the barriers between laboratories are low, so naturally collaborations happen across fields–interesting ideas may be hidden in everyday conversations.
*All the information on this page, including the researcher’s affiliation, is current at the time of the interview.
Paper information
One-Pot, Room-Temperature Conversion of CO2 into Porous Metal–Organic Frameworks
Kentaro Kadota, You-lee Hong, Yusuke Nishiyama, Easan Sivaniah, Daniel Packwood, and Satoshi Horike
Journal of the American Chemical Society
Published: October 2021
DOI: 10.1021/jacs.1c08227