Calcium: The Missing Link in Dead Cell Clean-up by Bridging Membrane Domains
Postdoctoral Fellow, Faculty of Science, Hokkaido University
PhD student with the Suzuki Lab, at the time of this research
Panpan Zhang
In our bodies, hundreds of billions of cells die daily and are cleared by phagocytes. To distinguish between dead cells and living cells, phosphatidylserine, a kind of lipid in the cell plasma membrane, acts as a critical feature by exposing on the surface of dead cells, leading to the dead cells being recognized and removed by phagocytes. In this study, Dr. Zhang and their colleagues have revealed the mechanism of phosphatidylserine exposure and uncovered a novel regulatory mechanism for modulating membrane proteins through calcium.

Please share with us the significance of your manuscript in terms of its research achievements, impact, or uniqueness.
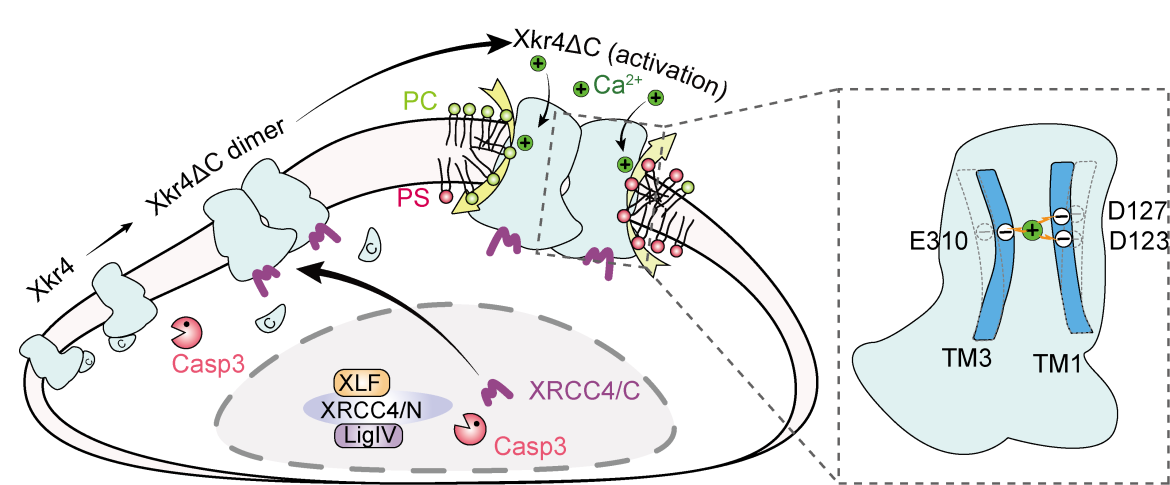
Xkr4 is a scramblase which facilitates phospholipid translocation across the plasma membrane bilayer. In previous studies at the Suzuki Lab, it was found that Xkr4 functions as dimer formation after cleavage at C-terminus by caspase and is activated by direct binding of XRCC4/C (C-terminus of XRCC4). However, dimer formation and XRCC4/C binding do not fully activate Xkr4. In this study, I have identified calcium as the critical factor for the activation of Xkr4.
Calcium acts as a secondary messenger in regulating protein functions. Typically, extracellular calcium binds to proteins on the extracellular side. However, our study found that the extracellular calcium binds to the transmembrane domain near the center of Xkr4, regulating the Xkr4 activation in the final step. This discovery reveals a novel regulatory mechanism for multi-transmembrane proteins.
Please tell us what was the most gratifying or inspiring moment for you during this research project.
Activation of scramblase causes changes in membrane tension. When considering how to demonstrate calcium binding to Xkr4, we got one idea by measuring the membrane tension in cells. Membrane tension is measured by applying physical force to pull beads attached to the cell membrane surface. However, since I used Baf3 cells, which are suspension cells, a challenge arose as they could not be attached to the dish surface tightly, leading to cells being easy to detach during prolonged measurement. It took over six months just to optimize the conditions for analysis before eventually getting the results. During this experiment I learned that success often requires great patience and perseverance in overcoming challenges.
Please tell us about the biggest challenge or problem you experienced while conducting your research. How did you overcome it?
The most challenging point of this study was understanding how calcium binding activates Xkr4. To measure the interaction of Xkr4 with calcium, I tried to purify Xkr4, but Xkr4 is unstable and difficult to purify! Eventually, I found one very cool approach. Our studies found that calcium was expected to bind to the negatively charged residues on transmembrane 1 and 3, functioning as a bridge to connect the two transmembrane domains. To emulate this calcium-mediated connection, I made mutations of these calcium-interacting residues. For instance, I utilized Cys mutations to introduce a disulfide bond, or positively charged Lys/Arg mutations to introduce a salt bridge with the calcium-interacting residue (the negatively charged residues: Asp/Glu). Using the artificial crosslinker, we successfully activated Xkr4 even in the absence of calcium. This method allows us to show that the calcium bindings to Xkr4 function as a molecular glue, facilitating the connection of the transmembrane domain in activated Xkr4.
Would you consider this work to be a turning point in your overall research direction? If so, how has your research direction changed as a result of this work?
Since joining the Suzuki Lab in 2018 for my PhD, I have been focused on studying scramblase to understand the connection between cell death and human health. This is a very interesting and important field, so I will still work in this field for my future research. Working in Suzuki Lab has provided me with invaluable experience in cell biology which will significantly help my future studies. Protein structure analysis has captivated me during my time as a master's student. Through the development of Cryo-electron microscopy, we can observe how proteins undergo changes and perform functions crucial for sustaining life. Next, I intend to explore membrane protein structure based on the knowledge which I learned here.
Please describe the current situation of your career. What is your current position? How has the knowledge and experience gained at iCeMS influenced your career?
I have moved to Faculty of Science, Hokkaido University as a Postdoc since Apr 2024. iCeMS is an outstanding international research platform that gathers researchers from around the globe, providing us with a great research environment and abundant resources. In addition, the on-site labs offer us more opportunities to collaborate with scholars from different countries. I'm grateful for the chance to visit the Center for Integrated Biosystems, iCeMS on-site lab in Taiwan. During my five years at iCeMS, a crucial phase in my research career as a doctoral candidate, I received comprehensive training and learned to approach my research with an international perspective. I believe that the experiences I gained here have equipped me with the necessary skills and confidence for my research career.
Finally, please give a message or advice to young iCeMS researchers.
Take things step by step and think by yourself, but do not hesitate to communicate with others, who you might get a good idea from.
Paper information
Extracellular calcium functions as a molecular glue for transmembrane helices to activate the scramblase Xkr4
Panpan Zhang, Masahiro Maruoka, Ryo Suzuki, Hikaru Katani, Yu Dou, Daniel M. Packwood, Hidetaka Kosako, Motomu Tanaka, and Jun Suzuki
Nature Communications
Published: September 2023
DOI: 10.1038/s41467-023-40934-2