Revival after a Century!
Mechanobiology has the potential to discover the secret of life
Bodies of living things, including mankind, consist of cells and tissues. How can a cell or tissue change its form or keep its structure? Life is still full of unknown secrets. There is a discipline that aims to elucidate the secret of life, known as "mechanobiology." This field of science, a challenge raised a century ago, has grown remarkably in this past decade, gradually elucidating the roles that physical forces play inside the body. In this issue, iCeMS researchers working in this challenging field introduce the fascinating points and prospects of mechanobiology.
Kaoru Sugimura
Program-Specific Research Center Associate Professor
Born in 1978 in Hyogo Prefecture. PhD, Graduate School of Science, Kyoto University. After working as a JSPS Research Fellow and RIKEN Postdoctoral Researcher, she served as an iCeMS Assistant Professor. In 2017, she took up her present post.

Naotaka Nakazawa
Program-Specific Assistant Professor
Born in 1984 in Gunma Prefecture. PhD, Graduate School of Industrial Science and Technology, Tokyo University of Science. He took up his present post in 2017 after serving as a Research Fellow at JSPS (DC1) and at the Mechanobiology Institute, National University of Singapore.

Why mechanics?
Mechanobiology is a field of science that studies how forces produced within and between cells affect the functions of cells and tissues. The key word is force. Mechanics take place in every part of a living body. For example, a cell can move by kicking its substrate by virtue of action and reaction forces. Eggs or fertilized eggs are transported in the fallopian tube, while foreign substances are expelled from the airway by the motion of cilia. Thus, without a mechanics perspective, you cannot scientifically explain events taking place in your own body. Someday, when the mechanics of life are elucidated in detail, we may understand the workings of the human body and throw light on the differences between living and non-living things.
On firm ground, you can run fast; while on muddy ground, you will be running slowly. This is also true with cells. Cells can move by kicking their substrate.
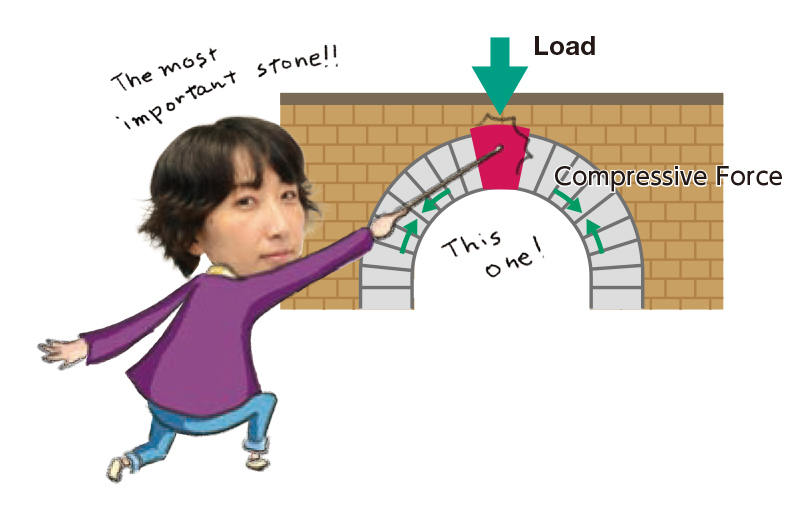
Starting About a Century Ago : History of Mechanobiology
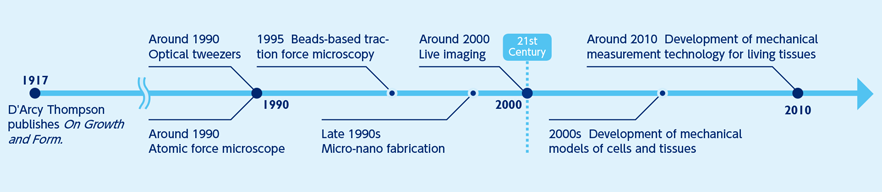
Although regarded as a new field of science, the origin of mechanobiology is not new, tracing back around a century ago. In 1917, the biologist D'Arcy Thompson published On Growth and Form. He proposed that physical or mathematical logic could explain the formation of the structures and patterns of animals and plants and that a living organism’s form was related to mechanical forces. Nevertheless, techniques at the time were inadequate for thorough research, and Thompson’s questions remained unaddressed for some time.
Around the turn of the century, many shifts in research perspective and technology occurred simultaneously in many fields of natural science including the development of mechanical measurement techniques, such as fluorescent imaging and micro-nano fabrication. These developments enabled researchers to quantitatively examine how mechanical forces organize the living system. Presently, knowledge and techniques from many fields are utilized to accelerate research activities. After a century, Thompson’s questions have been revived, and researchers are addressing these challenges in a renewed effort.
Seeing through the lens of mechanobiology
Observations of life from a mechanical perspective and using advanced technologies that evolved in the 21st century have enabled researchers to understand cells and tissues in new ways. Let us briefly see the world through the lens of mechanobiology. It may lead to discoveries beyond generally accepted ideas.
How do forces work in a body?
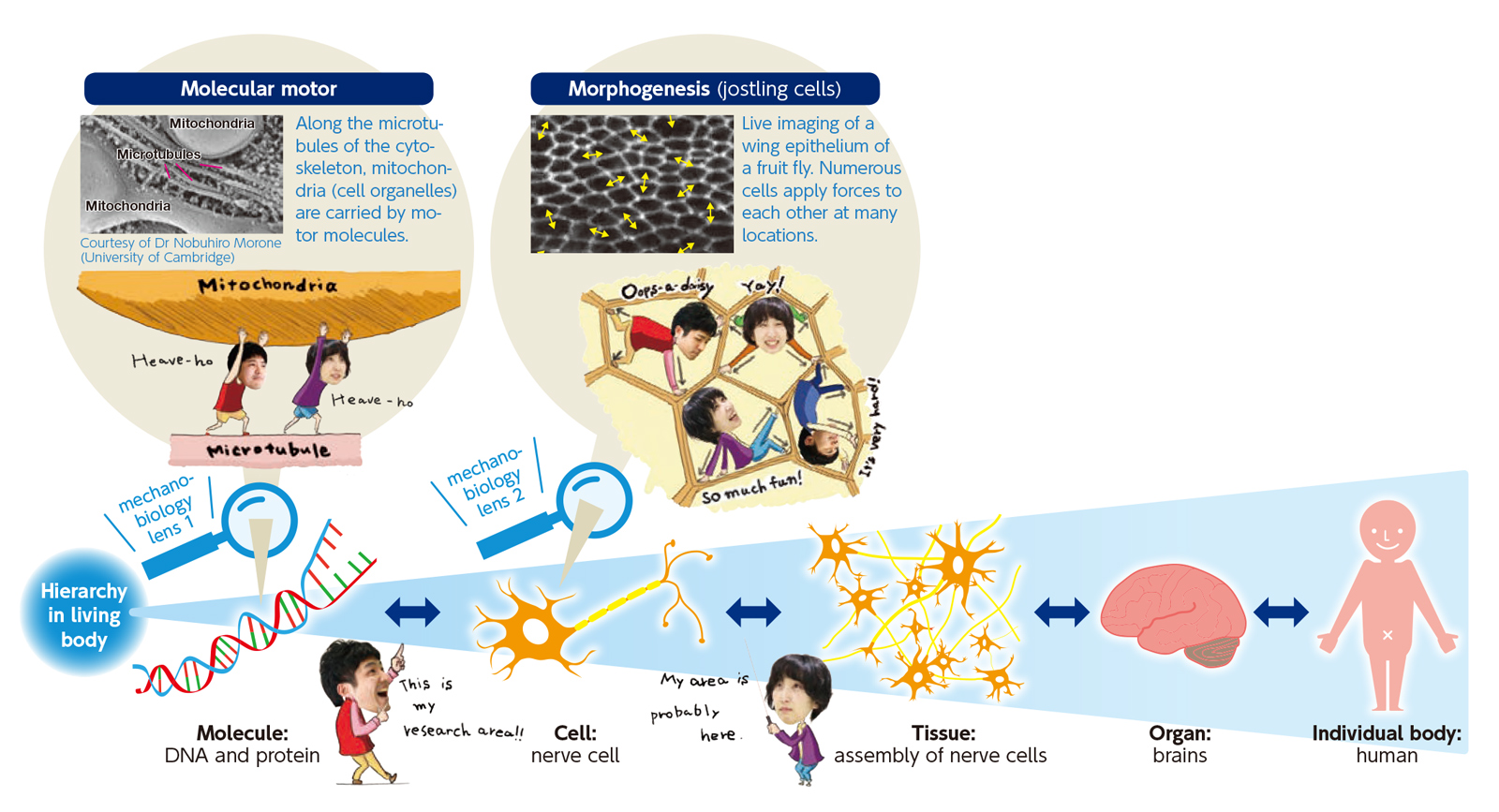
Approximately 37 trillion cells make up your body. Cells form tissues and organs, which in turn assemble into an individual body (human body). A hierarchical framework can represent the structure of a human body. In a cell, molecular motors generate forces, thereby deforming and moving the cell. Under these forces, cells jostle each other, acting on neighboring cells. By generating sensing, and reacting to forces, cells also influence the next hierarchical tier. Researchers in mechanobiology must understand the functions and structures of molecules and cells not only from a microscopic, but also from a multi-scale perspective.
The fate of a cell is determined by the mechanical environment
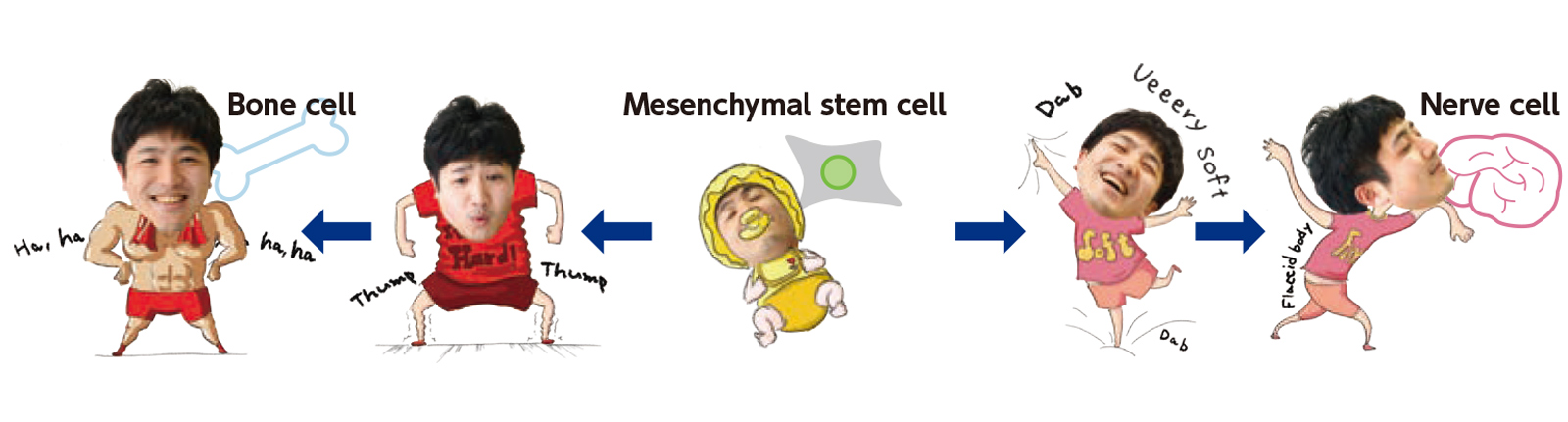
Cells not only sense forces, but they respond to the stiffness of the tissues around them. For example, mesenchymal stem cells differentiate into bone, muscle, or vascular endothelial cells and are highly regarded for their potential use in regenerative medicine. In 2006, it was found that substrate stiffness determines the type of cell into which these cells differentiate. For example, if the substrate is soft, the mesenchymal stem cell becomes a nerve cell; if hard, a bone cell; and if intermediate, a fat cell. The cell detects the substrate stiffness as a mechanical signal. Inside the cell, it is converted into a biochemical signal and transmitted to the nucleus. This process modulates gene expression to influence cell differentiation. In this way, the fate of a cell is determined by the mechanical characteristics of its environment.
How do you pursue research? — Research methods and techniques
In the study of mechanobiology, it is necessary to look into the behaviors of cells and molecules in great detail. Various methods and techniques are used for this research, some of which are described below from the three perspectives of observation, measurement, and manipulation.
Observation(live imaging)
Live imaging enables researchers to visualize and externally observe the dynamics of living tissues and cells and the action of gene expression. The key is to attach a green fluorescent protein (GFP) or a similar marker to a living cell or protein. Doing so allows for observation of the behavior of cells or proteins in living tissue using a fluorescent microscope or other instruments.
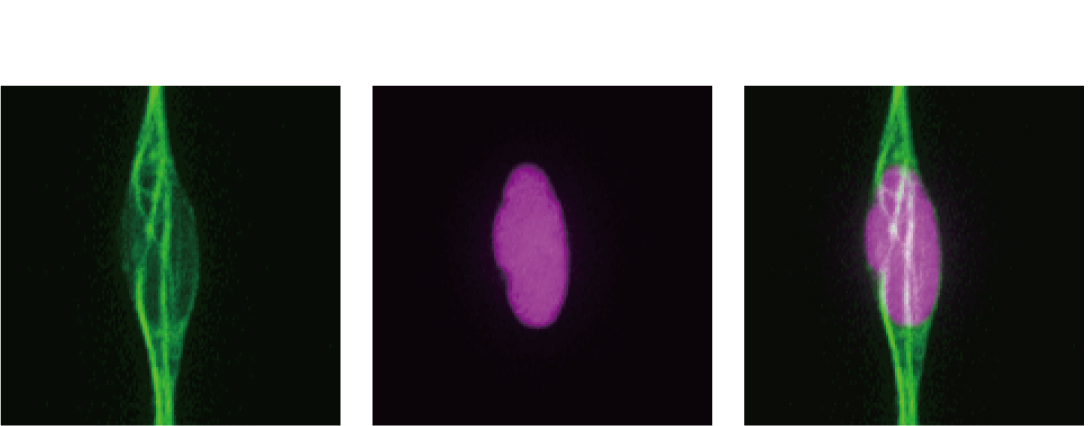
Left:microtubule (green); Middle: cell nucleus (magenta); Right: the superimposed image
Manipulation (microfabrication)
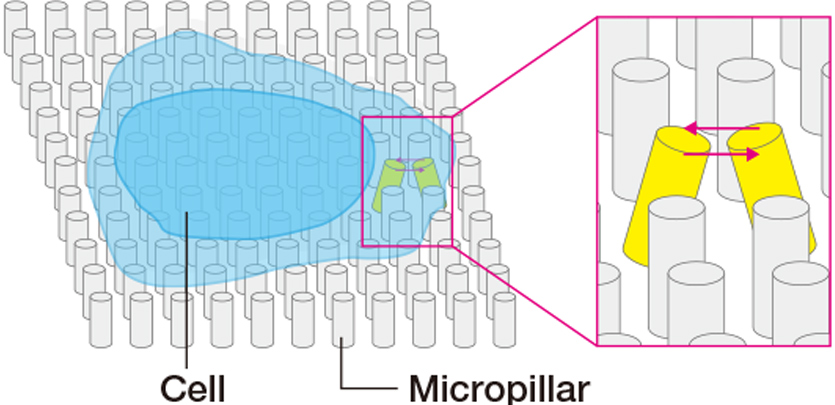
Polymer materials, such as polydimethylsiloxane and polyacrylamide, can bond cells when they are surface-coated with a protein that serves as a cellular scaffolding, In this process, it is possible to form scaffolding of various stiffness levels by varying the proportion of the cross-linking agent. Moreover, by combining the scaffolding with a microfabricated substrate, it becomes possible to change the cell adhesion pattern and form a channel for the passage of cells.

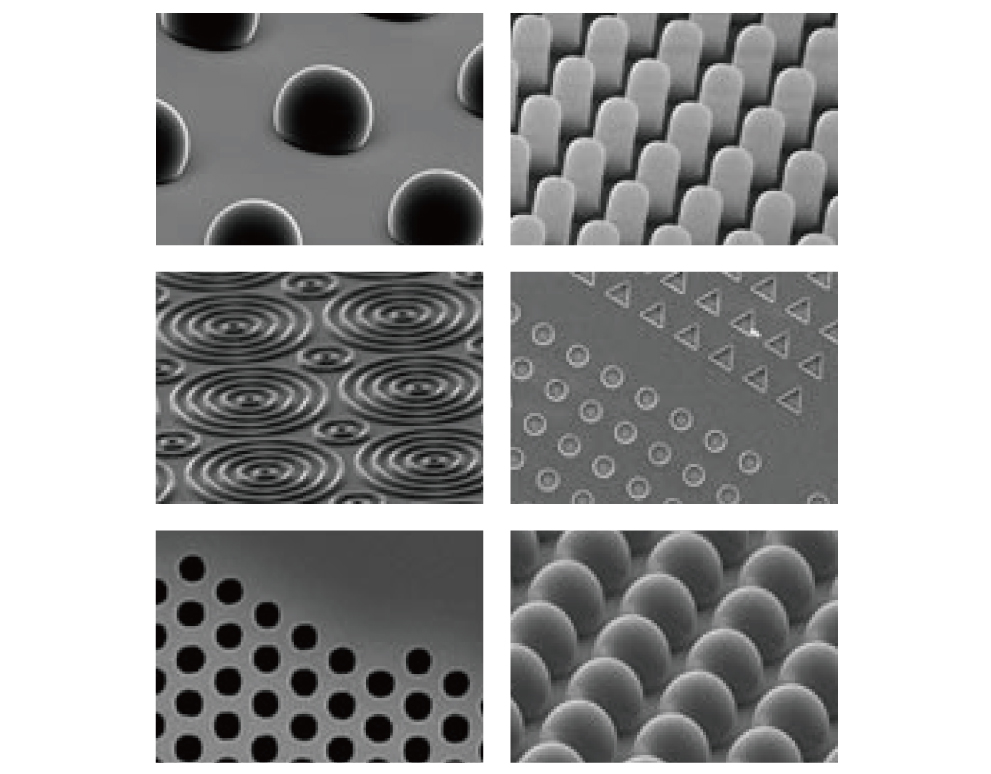
Courtesy of Dr Gianluca Grenci (Mechanobiology Institute, National University of Singapore)
Measurement
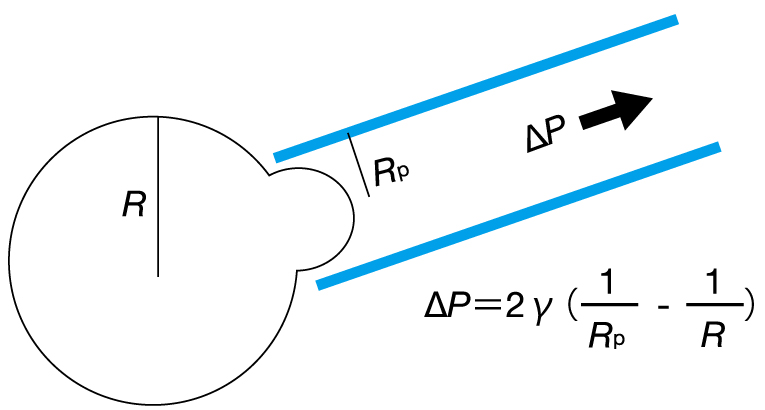
Direct contact with samples (micropipette technique)
When the micropipette tip contacts a cell or tissue sample and a negative pressure is applied, the sample is sucked up. Based on the sample deformation, the sample’s absolute value of surface tension is measured.
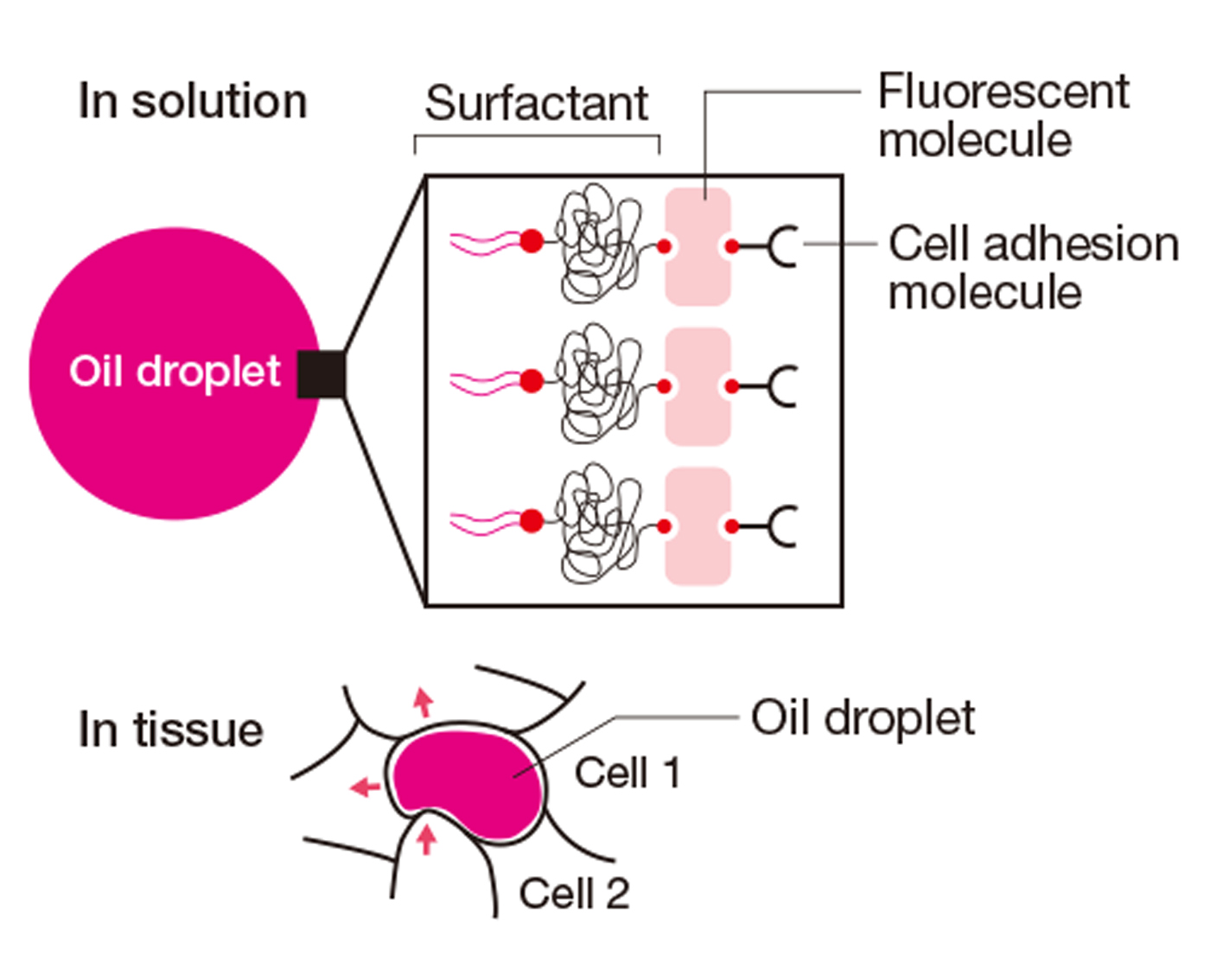
Measuring method by using a force sensor (oil droplet technique)
When an oil droplet is inserted between cells, the droplet is deformed due to the forces exerted by the neighboring cells. By previously measuring the surface tension of the oil droplet in a solution, the forces exerted by the neighboring cells can be calculated.
COLUMN

Quantitative Data
Information that can be measured, compiled, and analyzed with numbers are referred to as "quantitative data."
Full use of the above-described techniques enables obtaining quantitative data to analyze the complex workings of life. Scientific understanding of phenomena in mechanobiology, and in other fields as well, requires objective and detailed numbers that preclude subjective impressions and vague information. Researchers can construct theoretical models or discover unknown mechanisms of life by acquiring, comparing, and analyzing a vast amount of quantitative data on a daily basis.
iCeMS' Approach
At iCeMS, some researchers work on mechanobiology. They all similarly aim to elucidate the workings of life from a mechanics perspective. However, their focuses vary in terms of scale. The following is an overview of the research.
Motomu Tanaka
Specially Appointed Professor (iCeMS-CiMPhy)
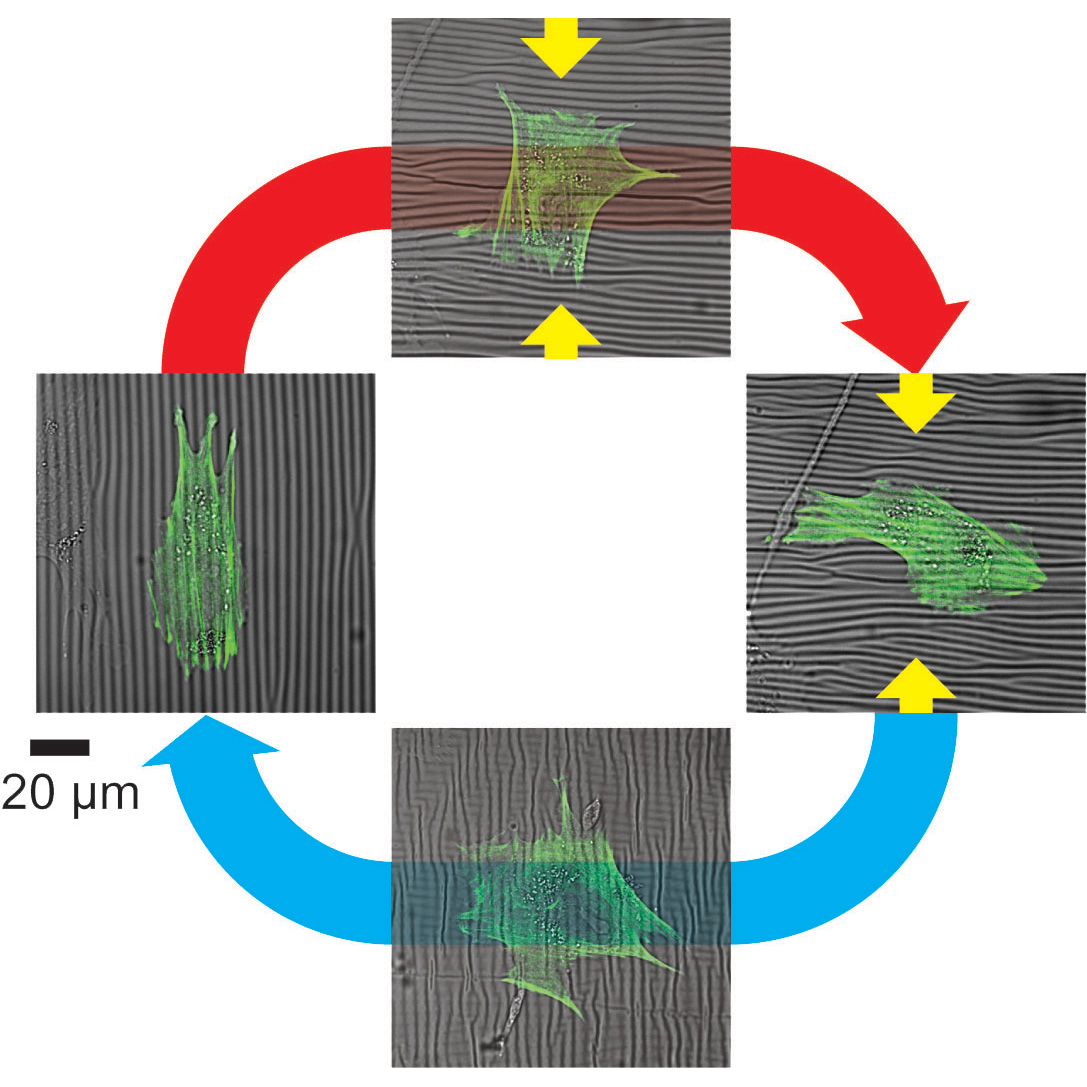
Tissues in our body, such as muscles, bones, and nerves, possess optimal levels of “stiffness/softness”. Cells sensitively feel how stiff/soft their surrounding environments (called extracellular matrices) are, and they adapt their shape and function accordingly. Physicists are not only good at measuring the stiffness of cells and extracellular environments, but also creating an innovative way to calculate the order of cells and tissues. With aid of materials that can flexibly change their stiffness and orientation, we tightly collaborate with medical doctors and biologists, and elucidate how dynamic changes in the cellular environment affect the fate of cells, such as healthy cells becoming sick.
Naotaka Nakazawa
In the brain development, each newborn neuron migrates from its birthplace to its functional place through very confined spaces formed by complex neurite configurations and extracellular matrices. Many mysteries remain unsolved in this process, such as how mechanical force contribute to neuronal migration in very narrow spaces and how neurons respond to mechanical stress by confined space during its migration. We work on these questions focusing on the nuclear dynamics during the neuronal migration in nervous tissue.

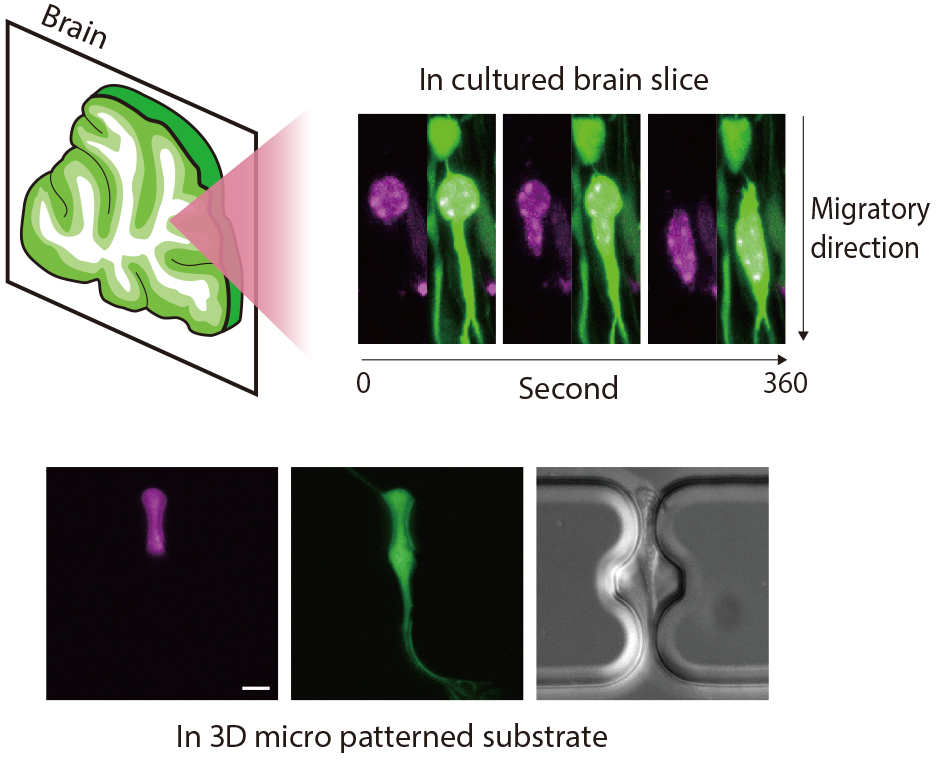
Bottom: Neuronal migration in micro patterned substrate with a confined space (Magenta: cell nucleus; Green: cytoplasm). This pattern is modulated by microfabrication techniques. Scale bar = 5 µm
Kaoru Sugimura
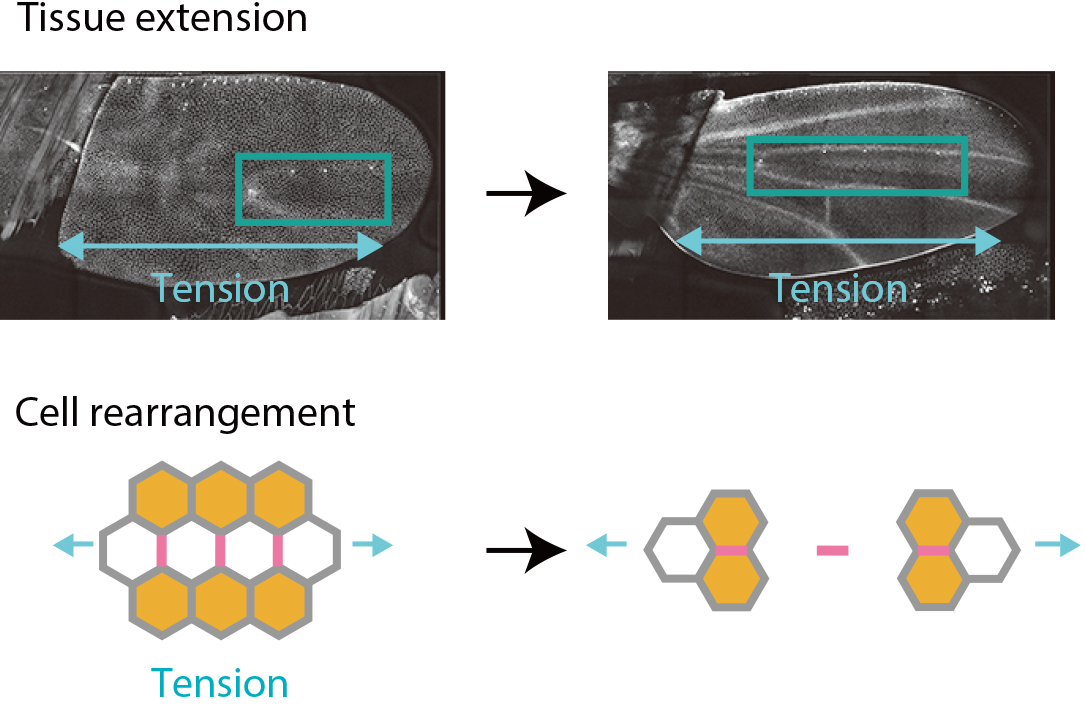
How do cells push and pull each other to trigger precise deformations of a tissue when shaping the body? The answer to this central question is essential for understanding the development of diverse forms in nature including our body. We have combined physics, mathematical statistics, and live imaging to infer forces acting between cells from the observed geometry of cells. By applying the method to fruit fly wing, we discovered that mechanical forces act as a messenger to transmit information between cells.

Published in March 2019 Reprinted from iCeMS Our World, Your Future vol.7
Production cooperation: Kyoto Tsushinsha