Calcium acts as missing link to dead cell clean-up
Scientists have found that extracellular calcium mediates the activation of a membrane protein that waves the flag signalling cell death.
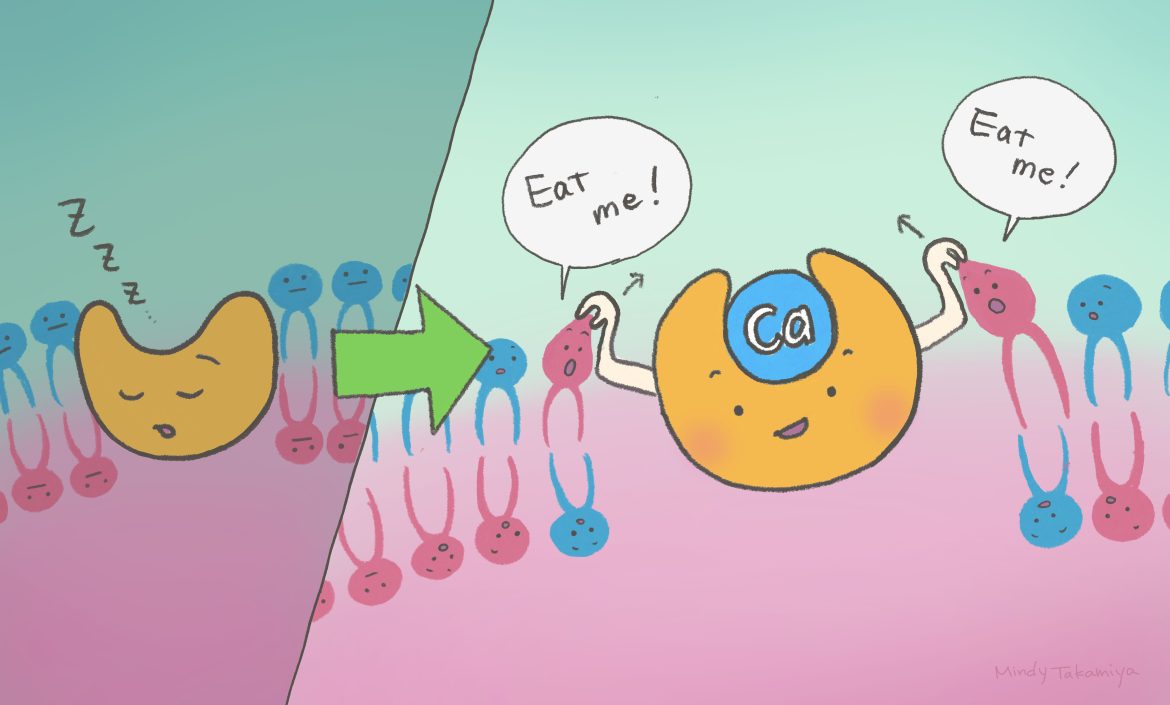
A research team co-led by scientists from Kyoto University Institute for Integrated Cell-Material Sciences (iCeMS) in Japan has uncovered mechanisms of how dying cells activate a protein that triggers an ‘eat me’ signal for immune cells to clean up the debris. The findings were published in Nature Communications.
The protein is called Xkr4, one of the Xkr family of proteins found in cell membranes. Xkr4 scrambles the phospholipid phosphatidylserine from the inner part of the cell membrane, where it normally resides, to its outside. Phosphatidylserine’s relocation to the outer part of the membrane is the signal that the cell is dying, and which attracts debris-gobbling phagocytes.
Researchers had previously discovered that, to act as a scramblase for phosphatidylserine, The C-terminal cytoplasmic tail of Xkr4 first has to be cleaved, forming a dimer with another Xkr4 and exposing a binding site. This binding site then connects to another protein fragment called XRCC4.
However, the binding of XRCC4 to Xkr4 alone is not enough to activate Xkr4 in the experimental setting. This suggested that another ingredient was required.
The research team in Japan discovered that calcium ions are required to enable activation of Xkr4. The positively charged calcium ions outside the cellular environment bind to three negatively charged amino acids on two ‘helices’ on the Xkr4 protein. This binding changes Xkr4 into a full-activated state from an intermediate state .
“We found that extracellular calcium functions as a molecular glue for Xkr4’s transmembrane helices, activating it,” explained iCeMS biochemist Jun Suzuki.
What’s surprising is that extracellular calcium is known to be involved in regulating the activity of proteins outside and inside the cellular environment, but not within the cell membrane itself. “Here, unexpectedly, we found that extracellular calcium is infiltrated into the transmembrane regions of proteins to connect two transmembrane helices,” Suzuki said.
The study also suggests that calcium ions might be important for activation of other members of the Xkr protein family, specifically Xkr8 and Xkr9, which could help clarify the mechanisms by which these and other scramblase proteins function.
The next step for the team is to examine the function of Xkr4 in nerve cells and explore their role in the brain.
Paper Information
“Extracellular calcium functions as a molecular glue for transmembrane helices to activate the
scramblase Xkr4”
Authors:Panpan Zhang, Masahiro Maruoka, Ryo Suzuki, Hikaru Katani, Yu Dou, Daniel M.
Packwood, Hidetaka Kosako, Motomu Tanaka, and Jun Suzuki







